当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role of the Chemically Non-Innocent Ligand in the Catalytic Formation of Hydrogen and Carbon Dioxide from Methanol and Water with the Metal as the Spectator
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2015-09-18 , DOI: 10.1021/jacs.5b07444 Haixia Li 1 , Michael B. Hall 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2015-09-18 , DOI: 10.1021/jacs.5b07444 Haixia Li 1 , Michael B. Hall 1
Affiliation
|
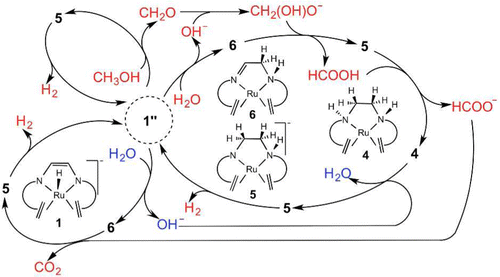
The catalytic mechanism for the production of H2 and CO2 from CH3OH and H2O by [K(dme)2][Ru(H) (trop2dad)] (K(dme)2.1_exp) was investigated by density functional theory (DFT) calculations. Since the reaction occurs under mild conditions and at reasonable rates, it could be considered an ideal way to use methanol to store hydrogen. The predicted mechanism begins with the dehydrogenation of methanol to formaldehyde through a new ligand-ligand bifunctional mechanism, where two hydrogen atoms of CH3OH eliminate to the ligand's N and C atoms, a mechanism that is more favorable than the previously known mechanisms, β-H elimination, or the metal-ligand bifunctional. The key initiator of this first step is formed by migration of the hydride in 1 from the ruthenium to the meta-carbon atom, which generates 1″ with a frustrated Lewis pair in the ring between N and C. Hydroxide, formed when 1″ cleaves H2O, reacts rapidly with CH2O to give H2C(OH)O(-), which subsequently donates a hydride to 6 to generate HCOOH and 5. HCOOH then protonates 5 to give formate and a neutral complex, 4, with a fully hydrogenated ligand. The hydride of formate transfers to 6, releasing CO2. The fully hydrogenated complex, 4, is first deprotonated by OH(-) to form 5, which then releases hydrogen to regenerate the catalyst, 1″. In this mechanism, which explains the experimental observations, the whole reaction occurs on the chemically non-innocent ligand with the ruthenium atom appearing as a spectator.
中文翻译:

化学非无害配体在以金属为旁观者的甲醇和水中催化生成氢气和二氧化碳中的作用
通过密度泛函理论 (DFT) 计算研究了 [K(dme)2][Ru(H) (trop2dad)] (K(dme)2.1_exp) 从 CH3OH 和 H2O 生产 H2 和 CO2 的催化机制。由于反应在温和的条件下以合理的速率发生,因此可以认为是使用甲醇储存氢气的理想方式。预测的机制开始于甲醇通过新的配体-配体双功能机制脱氢为甲醛,其中 CH3OH 的两个氢原子消除到配体的 N 和 C 原子,这种机制比以前已知的机制更有利,β-H消除,或金属配体双功能。第一步的关键引发剂是由氢化物从钌迁移到间位碳原子形成的,在 N 和 C 之间的环中生成 1" 与受挫的路易斯对。氢氧化物,当 1" 裂解 H2O 时形成,与 CH2O 快速反应生成 H2C(OH)O(-),随后将氢化物捐赠给 6 以生成HCOOH 和 5。然后 HCOOH 质子化 5,得到甲酸盐和中性配合物 4,具有完全氢化的配体。甲酸盐的氢化物转移到 6,释放出 CO2。完全氢化的配合物 4 首先被 OH(-) 去质子化以形成 5,然后释放氢以再生催化剂 1"。在这种解释实验观察的机制中,整个反应发生在化学非无害的配体上,钌原子作为旁观者出现。随后向 6 提供氢化物以生成 HCOOH 和 5。然后 HCOOH 将 5 质子化以得到甲酸盐和具有完全氢化配体的中性配合物 4。甲酸盐的氢化物转移到 6,释放出 CO2。完全氢化的配合物 4 首先被 OH(-) 去质子化以形成 5,然后释放氢以再生催化剂 1"。在这种解释实验观察的机制中,整个反应发生在化学非无害的配体上,钌原子作为旁观者出现。随后向 6 提供氢化物以生成 HCOOH 和 5。然后 HCOOH 将 5 质子化以得到甲酸盐和具有完全氢化配体的中性配合物 4。甲酸盐的氢化物转移到 6,释放出 CO2。完全氢化的配合物 4 首先被 OH(-) 去质子化以形成 5,然后释放氢以再生催化剂 1"。在这种解释实验观察的机制中,整个反应发生在化学非无害的配体上,钌原子作为旁观者出现。
更新日期:2015-09-18
中文翻译:

化学非无害配体在以金属为旁观者的甲醇和水中催化生成氢气和二氧化碳中的作用
通过密度泛函理论 (DFT) 计算研究了 [K(dme)2][Ru(H) (trop2dad)] (K(dme)2.1_exp) 从 CH3OH 和 H2O 生产 H2 和 CO2 的催化机制。由于反应在温和的条件下以合理的速率发生,因此可以认为是使用甲醇储存氢气的理想方式。预测的机制开始于甲醇通过新的配体-配体双功能机制脱氢为甲醛,其中 CH3OH 的两个氢原子消除到配体的 N 和 C 原子,这种机制比以前已知的机制更有利,β-H消除,或金属配体双功能。第一步的关键引发剂是由氢化物从钌迁移到间位碳原子形成的,在 N 和 C 之间的环中生成 1" 与受挫的路易斯对。氢氧化物,当 1" 裂解 H2O 时形成,与 CH2O 快速反应生成 H2C(OH)O(-),随后将氢化物捐赠给 6 以生成HCOOH 和 5。然后 HCOOH 质子化 5,得到甲酸盐和中性配合物 4,具有完全氢化的配体。甲酸盐的氢化物转移到 6,释放出 CO2。完全氢化的配合物 4 首先被 OH(-) 去质子化以形成 5,然后释放氢以再生催化剂 1"。在这种解释实验观察的机制中,整个反应发生在化学非无害的配体上,钌原子作为旁观者出现。随后向 6 提供氢化物以生成 HCOOH 和 5。然后 HCOOH 将 5 质子化以得到甲酸盐和具有完全氢化配体的中性配合物 4。甲酸盐的氢化物转移到 6,释放出 CO2。完全氢化的配合物 4 首先被 OH(-) 去质子化以形成 5,然后释放氢以再生催化剂 1"。在这种解释实验观察的机制中,整个反应发生在化学非无害的配体上,钌原子作为旁观者出现。随后向 6 提供氢化物以生成 HCOOH 和 5。然后 HCOOH 将 5 质子化以得到甲酸盐和具有完全氢化配体的中性配合物 4。甲酸盐的氢化物转移到 6,释放出 CO2。完全氢化的配合物 4 首先被 OH(-) 去质子化以形成 5,然后释放氢以再生催化剂 1"。在这种解释实验观察的机制中,整个反应发生在化学非无害的配体上,钌原子作为旁观者出现。













































 京公网安备 11010802027423号
京公网安备 11010802027423号