Joule ( IF 35.4 ) Pub Date : 2017-11-01 , DOI: 10.1016/j.joule.2017.10.008
Xiaohui Rong , Jue Liu , Enyuan Hu , Yijin Liu , Yi Wang , Jinpeng Wu , Xiqian Yu , Katharine Page , Yong-Sheng Hu , Wanli Yang , Hong Li , Xiao-Qing Yang , Liquan Chen , Xuejie Huang
|
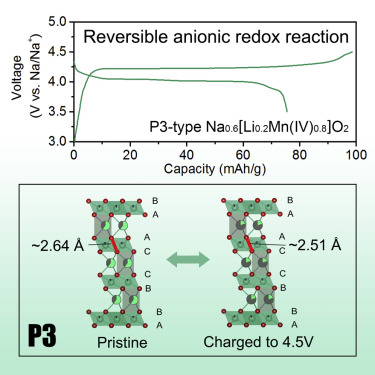
Anionic redox reaction (ARR) in lithium- and sodium-ion batteries is under hot discussion, mainly regarding how oxygen anion participates and to what extent oxygen can be reversibly oxidized and reduced. Here, a P3-type Na0.6[Li0.2Mn0.8]O2 with reversible capacity from pure ARR was studied. The interlayer O-O distance (peroxo-like O-O dimer, 2.506(3) Å), associated with oxidization of oxygen anions, was directly detected by using a neutron total scattering technique. Different from Li2RuO3 or Li2IrO3 with strong metal-oxygen (M-O) bonding, for P3-type Na0.6[Li0.2Mn0.8]O2 with relatively weak Mn-O covalent bonding, crystal structure factors might play an even more important role in stabilizing the oxidized species, as both Li and Mn ions are immobile in the structure and thus may inhibit the irreversible transformation of the oxidized species to O2 gas.
中文翻译:

Na层状氧化物阴极中结构诱导的可逆阴离子氧化还原活性
锂离子电池和钠离子电池中的阴离子氧化还原反应(ARR)受到热议,主要涉及氧阴离子如何参与以及氧可逆氧化和还原的程度。在此,研究了纯ARR具有可逆容量的P3型Na 0.6 [Li 0.2 Mn 0.8 ] O 2。通过使用中子全散射技术直接检测与氧阴离子的氧化有关的层间OO距离(类过氧化物的OO二聚体,2.506(3)Å)。选自Li不同2的RuO 3或Li 2的IrO 3具有较强的金属-氧(MO)键合,对于P3型的Na 0.6 [李0.2锰0.8具有相对较弱的Mn-O共价键的] O 2,晶体结构因子可能在稳定氧化物种中起着更为重要的作用,因为Li和Mn离子均不固定在结构中,因此可以抑制氧化物种的不可逆转变到O 2气。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号
