当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Porous Chitin Microbeads for More Sustainable Cosmetics†
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1021/acssuschemeng.7b03053 Catherine A. King 1 , Julia L. Shamshina 2 , Oleksandra Zavgorodnya 3 , Tatum Cutfield 1 , Leah E. Block 3 , Robin D. Rogers 1, 3, 4
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1021/acssuschemeng.7b03053 Catherine A. King 1 , Julia L. Shamshina 2 , Oleksandra Zavgorodnya 3 , Tatum Cutfield 1 , Leah E. Block 3 , Robin D. Rogers 1, 3, 4
Affiliation
|
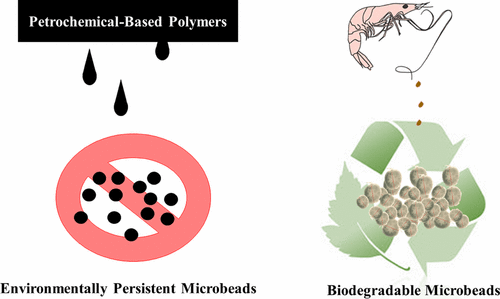
The microbead form is a material architecture that is promising for use in biomedical and cosmetic applications; however, the use of petroleum-based microbeads (i.e., plastics) has raised significant environmental concerns in recent years. Microbeads prepared from renewable polymers could represent a sustainable alternative to these synthetic microbeads. This work explores the use of chitin in preparing biodegradable, biocompatible microbeads of low toxicity. Chitin microbeads were synthesized using the ionic liquid (IL) 1-ethyl-3-methylimidazolium acetate ([C2mim][OAc]); the IL was used to both extract chitin directly from waste shrimp shell and to prepare the porous microbeads by coagulation in polypropylene glycol (PPG). The effects of biopolymer source and bead-preparation parameters on the formation of beads were investigated, as well as the effects of the drying conditions on the dry bead structure. It was found that IL-extracted chitin could be used to prepare beads of homogeneous size distribution (with 60% of beads 125–250 μm) and shape, while commercially available practical grade chitin could not, suggesting that high molecular weight chitin is required for bead-material formation. Supercritical CO2 drying and lyophilization of the wet beads led to dry chitin beads with an opaque appearance, porous interiors, and uniform shape. Loading and release studies of representative active compounds (indigo dye and sodium salicylate) into the chitin beads indicated that the dry beads could be easily loaded from an aqueous solution of active compound and could release 90% of the active compound within 7 h in deionized (DI) water at room temperature.
中文翻译:

多孔几丁质微珠可实现更具可持续性的化妆品†
微珠形式是一种有望用于生物医学和化妆品应用的材料结构。然而,近年来使用石油基微珠(即塑料)引起了人们对环境的重大关注。由可再生聚合物制备的微珠可以代表这些合成微珠的可持续替代品。这项工作探索了甲壳素在制备低毒性的可生物降解,生物相容性微珠中的用途。使用离子液体(IL)1-乙基-3-甲基咪唑鎓乙酸盐([C 2mim] [OAc]); IL既可以直接从废虾壳中提取几丁质,又可以通过在聚丙二醇(PPG)中凝结来制备多孔微珠。研究了生物聚合物来源和珠子制备参数对珠子形成的影响,以及干燥条件对干珠结构的影响。研究发现,IL提取的几丁质可用于制备大小均一的珠子(60%的珠子为125–250μm)和形状,而市售的实用级几丁质则不能,这表明高分子量的几丁质是必需的。磁珠材料的形成。超临界CO 2湿珠的干燥和冻干导致干燥的甲壳质珠具有不透明的外观,多孔的内部结构和均匀的形状。代表性活性化合物(靛蓝染料和水杨酸钠)在几丁质珠粒中的负载和释放研究表明,干燥的珠粒可以轻松地从活性化合物的水溶液中负载,并且可以在去离子(7h)后的7小时内释放90%的活性化合物。 DI)室温下的水。
更新日期:2017-11-09
中文翻译:

多孔几丁质微珠可实现更具可持续性的化妆品†
微珠形式是一种有望用于生物医学和化妆品应用的材料结构。然而,近年来使用石油基微珠(即塑料)引起了人们对环境的重大关注。由可再生聚合物制备的微珠可以代表这些合成微珠的可持续替代品。这项工作探索了甲壳素在制备低毒性的可生物降解,生物相容性微珠中的用途。使用离子液体(IL)1-乙基-3-甲基咪唑鎓乙酸盐([C 2mim] [OAc]); IL既可以直接从废虾壳中提取几丁质,又可以通过在聚丙二醇(PPG)中凝结来制备多孔微珠。研究了生物聚合物来源和珠子制备参数对珠子形成的影响,以及干燥条件对干珠结构的影响。研究发现,IL提取的几丁质可用于制备大小均一的珠子(60%的珠子为125–250μm)和形状,而市售的实用级几丁质则不能,这表明高分子量的几丁质是必需的。磁珠材料的形成。超临界CO 2湿珠的干燥和冻干导致干燥的甲壳质珠具有不透明的外观,多孔的内部结构和均匀的形状。代表性活性化合物(靛蓝染料和水杨酸钠)在几丁质珠粒中的负载和释放研究表明,干燥的珠粒可以轻松地从活性化合物的水溶液中负载,并且可以在去离子(7h)后的7小时内释放90%的活性化合物。 DI)室温下的水。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号