当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cucurbit[7]uril-Driven Host–Guest Chemistry for Reversible Intervention of 5-Formylcytosine-Targeted Biochemical Reactions
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-11-09 00:00:00 , DOI: 10.1021/jacs.7b09635 Shao-Ru Wang 1 , Yan-Yan Song 1 , Lai Wei 1 , Chao-Xing Liu 1 , Bo-Shi Fu 1 , Jia-Qi Wang 1 , Xi-Ran Yang 2 , Yi-Nong Liu 1 , Si-Min Liu 2 , Tian Tian 1 , Xiang Zhou 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-11-09 00:00:00 , DOI: 10.1021/jacs.7b09635 Shao-Ru Wang 1 , Yan-Yan Song 1 , Lai Wei 1 , Chao-Xing Liu 1 , Bo-Shi Fu 1 , Jia-Qi Wang 1 , Xi-Ran Yang 2 , Yi-Nong Liu 1 , Si-Min Liu 2 , Tian Tian 1 , Xiang Zhou 1
Affiliation
|
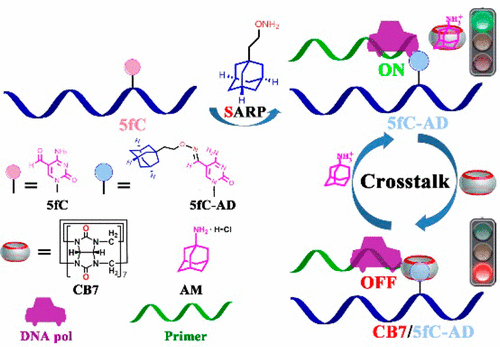
5-Formylcytosine (5fC) is identified as one of the key players in active DNA demethylation and also as an epigenetic mark in mammals, thus representing a novel attractive target to chemical intervention. The current study represents an attempt to develop a reversible 5fC-targeted intervention tool. A supramolecular aldehyde reactive probe was therefore introduced for selective conversion of the 5fC to 5fC-AD nucleotide. Using various methods, we demonstrate that cucurbit[7]uril (CB7) selectively targets the 5fC-AD nucleotide in DNA, however, the binding of CB7 to 5fC-AD does not affect the hydrogen bonding properties of natural nucleobases in duplex DNA. Importantly, CB7-driven host–guest chemistry has been applied for reversible intervention of a variety of 5fC-targeted biochemical reactions, including restriction endonuclease digestion, DNA polymerase elongation, and polymerase chain reaction. On the basis of the current study, the macrocyclic CB7 creates obstructions that, through steric hindrance, prevent the enzyme from binding to the substrate, whereas the CB7/5fC-AD host–guest interactions can be reversed by treatment with adamantanamine. Moreover, fragment- and site-specific identification of 5fC modification in DNA has been accomplished without sequence restrictions. These findings thus show promising potential of host–guest chemistry for DNA/RNA epigenetics.
中文翻译:

葫芦[7]尿嘧啶驱动的宿主-来宾化学对5-甲酰胞嘧啶靶向的生化反应的可逆干预
5-甲酰基胞嘧啶(5fC)被认为是活跃的DNA去甲基化的关键参与者之一,并且在哺乳动物中也被视为表观遗传标记,因此代表了化学干预的一个新的有吸引力的靶标。当前的研究代表了开发可逆的以5fC为目标的干预工具的尝试。因此引入了超分子醛反应性探针,以将5fC选择性转化为5fC-AD核苷酸。使用各种方法,我们证明葫芦[7]尿嘧啶(CB7)选择性靶向DNA中的5fC-AD核苷酸,但是,CB7与5fC-AD的结合不会影响双链DNA中天然核碱基的氢键键合特性。重要的是,CB7驱动的宿主-客体化学已用于可逆性干预多种5fC靶向的生化反应,包括限制性核酸内切酶消化,DNA聚合酶的延伸,与聚合酶链反应有关。根据当前的研究,大环CB7会产生阻碍,通过空间位阻可阻止酶与底物结合,而CB7 / 5fC-AD宿主与客体的相互作用可通过金刚烷胺的处理而逆转。而且,已经完成了DNA中5fC修饰的片段和位点特异性鉴定,而没有序列限制。因此,这些发现显示了宿主/客体化学在DNA / RNA表观遗传学方面的潜力。DNA中5fC修饰的片段和位点特异性鉴定已经完成,没有序列限制。因此,这些发现显示了宿主/客体化学在DNA / RNA表观遗传学方面的潜力。DNA中5fC修饰的片段和位点特异性鉴定已经完成,没有序列限制。因此,这些发现显示了宿主/客体化学在DNA / RNA表观遗传学方面的潜力。
更新日期:2017-11-09
中文翻译:

葫芦[7]尿嘧啶驱动的宿主-来宾化学对5-甲酰胞嘧啶靶向的生化反应的可逆干预
5-甲酰基胞嘧啶(5fC)被认为是活跃的DNA去甲基化的关键参与者之一,并且在哺乳动物中也被视为表观遗传标记,因此代表了化学干预的一个新的有吸引力的靶标。当前的研究代表了开发可逆的以5fC为目标的干预工具的尝试。因此引入了超分子醛反应性探针,以将5fC选择性转化为5fC-AD核苷酸。使用各种方法,我们证明葫芦[7]尿嘧啶(CB7)选择性靶向DNA中的5fC-AD核苷酸,但是,CB7与5fC-AD的结合不会影响双链DNA中天然核碱基的氢键键合特性。重要的是,CB7驱动的宿主-客体化学已用于可逆性干预多种5fC靶向的生化反应,包括限制性核酸内切酶消化,DNA聚合酶的延伸,与聚合酶链反应有关。根据当前的研究,大环CB7会产生阻碍,通过空间位阻可阻止酶与底物结合,而CB7 / 5fC-AD宿主与客体的相互作用可通过金刚烷胺的处理而逆转。而且,已经完成了DNA中5fC修饰的片段和位点特异性鉴定,而没有序列限制。因此,这些发现显示了宿主/客体化学在DNA / RNA表观遗传学方面的潜力。DNA中5fC修饰的片段和位点特异性鉴定已经完成,没有序列限制。因此,这些发现显示了宿主/客体化学在DNA / RNA表观遗传学方面的潜力。DNA中5fC修饰的片段和位点特异性鉴定已经完成,没有序列限制。因此,这些发现显示了宿主/客体化学在DNA / RNA表观遗传学方面的潜力。































 京公网安备 11010802027423号
京公网安备 11010802027423号