当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Utilization of Desalination Brine for Sodium Hydroxide Production: Technologies, Engineering Principles, Recovery Limits, and Future Directions
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acssuschemeng.7b02276 Gregory P. Thiel 1 , Amit Kumar 1 , Alicia Gómez-González 2 , John H. Lienhard 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acssuschemeng.7b02276 Gregory P. Thiel 1 , Amit Kumar 1 , Alicia Gómez-González 2 , John H. Lienhard 1
Affiliation
|
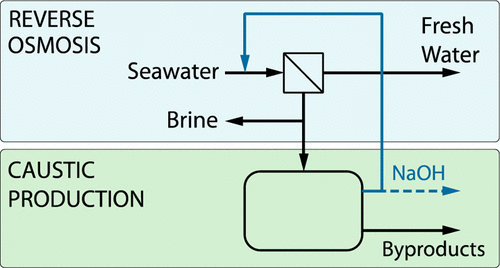
As global desalination capacity continues its rapid growth, the impetus for reducing the adverse environmental impacts of brine discharge grows concurrently. Although modern brine outfall designs have significantly limited such impacts, they are costly. Recovering valuable components and chemical derivatives from brine has potential to resolve both environmental and economic concerns. In this article, methods for producing sodium hydroxide (“caustic”) from seawater reverse osmosis (SWRO) brine for internal reuse, which typically involve brine purification, brine concentration, and sodium chloride electrolysis, are reviewed. Because process energy consumption drives process cost and caustic purity determines product usability in drinking water systems, reviewed technologies are benchmarked against thermodynamic minimum energy consumption and maximum (stoichiometric) NaOH production rates. After individual reviews of brine purification, concentration, and electrolysis technologies, five existing facilities for caustic production from seawater and seawater concentrates are discussed. Bipolar membrane electrodialysis appears to have the best potential to meet the technoeconomic requirements of small-scale caustic production from SWRO brine. Finally, future research and demonstration needs, to bring the technology to commercial feasibility, are identified.
中文翻译:

利用淡化盐水生产氢氧化钠:技术,工程原理,回收率限制和未来方向
随着全球海水淡化能力的持续快速增长,减少盐水排放对环境的不利影响的动力也随之增长。尽管现代盐水排污口设计已大大限制了此类影响,但它们的成本很高。从盐水中回收有价值的成分和化学衍生物具有解决环境和经济问题的潜力。在本文中,回顾了从海水反渗透(SWRO)盐水生产氢氧化钠(“苛性碱”)以供内部重复使用的方法,这些方法通常涉及盐水净化,盐水浓缩和氯化钠电解。由于过程能源消耗决定了过程成本,而苛性碱纯度决定了饮用水系统中产品的可用性,经过审查的技术以热力学的最低能耗和最大(化学计量)NaOH生产率为基准。在对盐水的净化,浓缩和电解技术进行单独评论之后,讨论了五个现有的利用海水和海水浓缩液进行苛性碱生产的设施。双极性膜电渗析似乎最有可能满足由SWRO盐水进行小规模苛性碱生产的技术经济要求。最后,确定了将技术应用于商业的未来研究和示范需求。双极性膜电渗析似乎最有可能满足由SWRO盐水进行小规模苛性碱生产的技术经济要求。最后,确定了将技术应用于商业的未来研究和示范需求。双极性膜电渗析似乎最有可能满足由SWRO盐水进行小规模苛性碱生产的技术经济要求。最后,确定了将技术应用于商业的未来研究和示范需求。
更新日期:2017-11-07
中文翻译:

利用淡化盐水生产氢氧化钠:技术,工程原理,回收率限制和未来方向
随着全球海水淡化能力的持续快速增长,减少盐水排放对环境的不利影响的动力也随之增长。尽管现代盐水排污口设计已大大限制了此类影响,但它们的成本很高。从盐水中回收有价值的成分和化学衍生物具有解决环境和经济问题的潜力。在本文中,回顾了从海水反渗透(SWRO)盐水生产氢氧化钠(“苛性碱”)以供内部重复使用的方法,这些方法通常涉及盐水净化,盐水浓缩和氯化钠电解。由于过程能源消耗决定了过程成本,而苛性碱纯度决定了饮用水系统中产品的可用性,经过审查的技术以热力学的最低能耗和最大(化学计量)NaOH生产率为基准。在对盐水的净化,浓缩和电解技术进行单独评论之后,讨论了五个现有的利用海水和海水浓缩液进行苛性碱生产的设施。双极性膜电渗析似乎最有可能满足由SWRO盐水进行小规模苛性碱生产的技术经济要求。最后,确定了将技术应用于商业的未来研究和示范需求。双极性膜电渗析似乎最有可能满足由SWRO盐水进行小规模苛性碱生产的技术经济要求。最后,确定了将技术应用于商业的未来研究和示范需求。双极性膜电渗析似乎最有可能满足由SWRO盐水进行小规模苛性碱生产的技术经济要求。最后,确定了将技术应用于商业的未来研究和示范需求。































 京公网安备 11010802027423号
京公网安备 11010802027423号