当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Impact of the US FDA “Biopharmaceutics Classification System” (BCS) Guidance on Global Drug Development
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00687 Mehul U. Mehta 1 , Ramana S. Uppoor 1 , Dale P Conner 1 , Paul Seo 1 , Jayabharathi Vaidyanathan 1 , Donna A. Volpe 1 , Ethan Stier 1 , Dakshina Chilukuri 1 , Angelica Dorantes 1 , Tapash Ghosh 1 , Haritha Mandula 1 , Kimberly Raines 1 , Pariban Dhanormchitphong 1 , Janet Woodcock 1 , Lawrence X Yu 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00687 Mehul U. Mehta 1 , Ramana S. Uppoor 1 , Dale P Conner 1 , Paul Seo 1 , Jayabharathi Vaidyanathan 1 , Donna A. Volpe 1 , Ethan Stier 1 , Dakshina Chilukuri 1 , Angelica Dorantes 1 , Tapash Ghosh 1 , Haritha Mandula 1 , Kimberly Raines 1 , Pariban Dhanormchitphong 1 , Janet Woodcock 1 , Lawrence X Yu 1
Affiliation
|
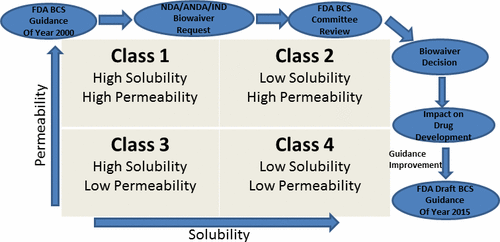
The FDA guidance on application of the biopharmaceutics classification system (BCS) for waiver of in vivo bioequivalence (BE) studies was issued in August 2000. Since then, this guidance has created worldwide interest among biopharmaceutical scientists in regulatory agencies, academia, and industry toward its implementation and further expansion. This article describes how the review implementation of this guidance was undertaken at the FDA and results of these efforts over last dozen years or so across the new, and the generic, drug domains are provided. Results show that greater than 160 applications were approved, or tentatively approved, based on the BCS approach across multiple therapeutic areas; an additional significant finding was that at least 50% of these approvals were in the central nervous system (CNS) area. These findings indicate a robust utilization of the BCS approach toward reducing unnecessary in vivo BE studies and speeding up availability of high quality pharmaceutical products. The article concludes with a look at the adoption of this framework by regulatory and health policy organizations across the globe, and FDA’s current thinking on areas of improvement of this guidance.
中文翻译:

美国FDA“生物制药分类系统”(BCS)指南对全球药物开发的影响
FDA于2000年8月发布了有关使用生物制药分类系统(BCS)放弃体内生物等效性(BE)研究的指南。此后,该指南引起了监管机构,学术界和工业界对生物制药科学家的广泛关注。其实施和进一步扩展。本文介绍了如何在FDA进行本指南的审查实施,以及在过去十几年左右的时间里在新的和通用的药物领域中所做的努力的结果。结果表明,基于BCS方法在多个治疗领域中已批准或初步批准了160多个申请;另一个重要发现是,这些批准中至少有50%位于中枢神经系统(CNS)区域。这些发现表明,BCS方法得到了强有力的利用,以减少不必要的体内BE研究并加快了高质量药物产品的可用性。本文以全球监管和卫生政策组织采用此框架作为结束语,并介绍了FDA当前在本指南改进领域上的想法。
更新日期:2017-11-08
中文翻译:

美国FDA“生物制药分类系统”(BCS)指南对全球药物开发的影响
FDA于2000年8月发布了有关使用生物制药分类系统(BCS)放弃体内生物等效性(BE)研究的指南。此后,该指南引起了监管机构,学术界和工业界对生物制药科学家的广泛关注。其实施和进一步扩展。本文介绍了如何在FDA进行本指南的审查实施,以及在过去十几年左右的时间里在新的和通用的药物领域中所做的努力的结果。结果表明,基于BCS方法在多个治疗领域中已批准或初步批准了160多个申请;另一个重要发现是,这些批准中至少有50%位于中枢神经系统(CNS)区域。这些发现表明,BCS方法得到了强有力的利用,以减少不必要的体内BE研究并加快了高质量药物产品的可用性。本文以全球监管和卫生政策组织采用此框架作为结束语,并介绍了FDA当前在本指南改进领域上的想法。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号