JAMA Neurology ( IF 20.4 ) Pub Date : 2018-01-01 , DOI: 10.1001/jamaneurol.2017.3171 Fabian Büchele 1 , Marc Hackius 1 , Sebastian R. Schreglmann 1 , Wolfgang Omlor 1 , Esther Werth 1 , Angelina Maric 1 , Lukas L. Imbach 1 , Stefan Hägele-Link 2 , Daniel Waldvogel 1 , Christian R. Baumann 1
|
Importance Sleep-wake disorders are a common and debilitating nonmotor manifestation of Parkinson disease (PD), but treatment options are scarce.
Objective To determine whether nocturnal administration of sodium oxybate, a first-line treatment in narcolepsy, is effective and safe for excessive daytime sleepiness (EDS) and disturbed nighttime sleep in patients with PD.
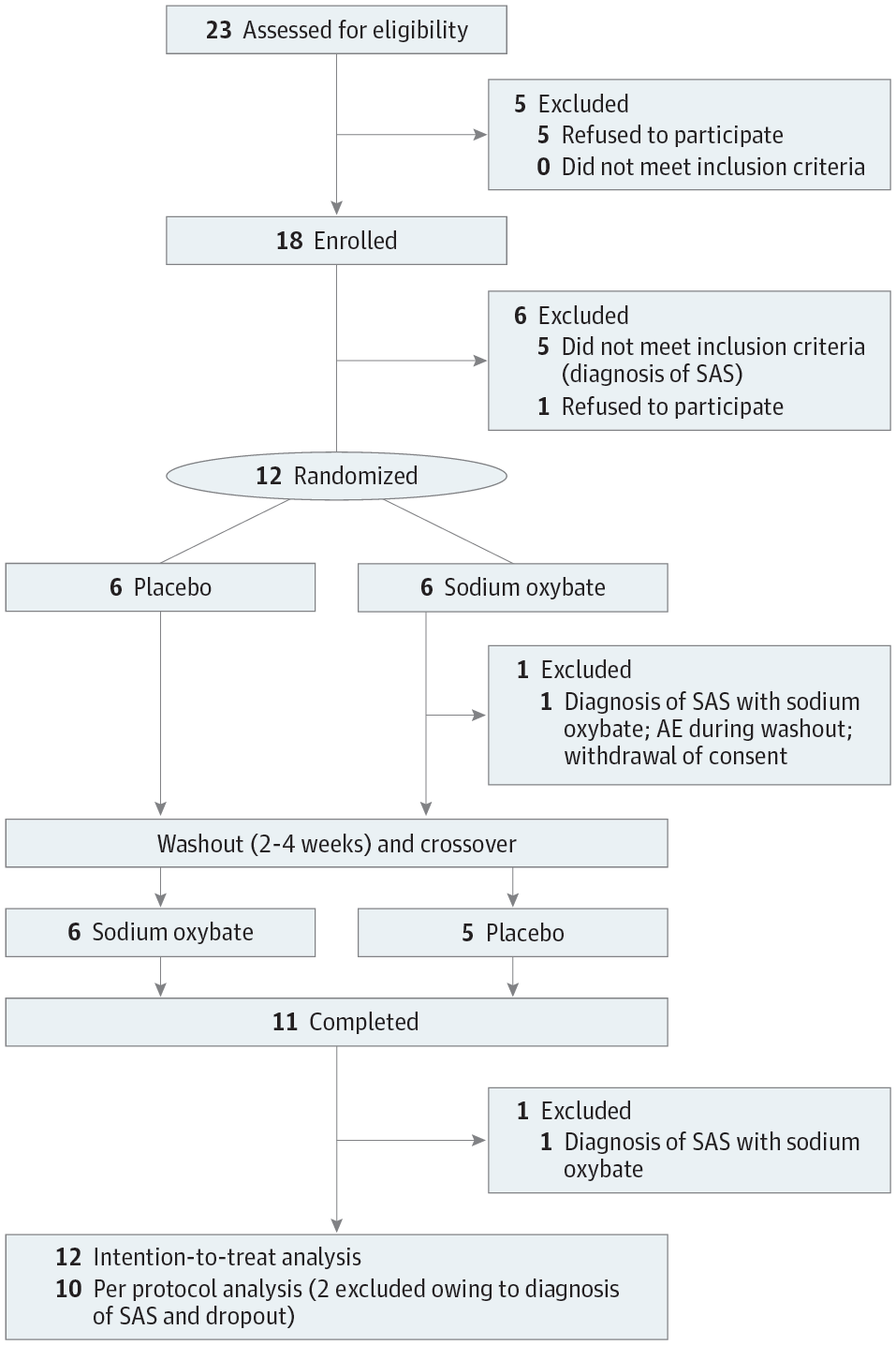
Design, Setting, and Participants Randomized, double-blind, placebo-controlled, crossover phase 2a study carried out between January 9, 2015, and February 24, 2017. In a single-center study in the sleep laboratory at the University Hospital Zurich, Zurich, Switzerland, 18 patients with PD and EDS (Epworth Sleepiness Scale [ESS] score >10) were screened in the sleep laboratory. Five patients were excluded owing to the polysomnographic diagnosis of sleep apnea and 1 patient withdrew consent. Thus, 12 patients were randomized to a treatment sequence (sodium oxybate followed by placebo or placebo followed by sodium oxybate, ratio 1:1) and, after dropout of 1 patient owing to an unrelated adverse event during the washout period, 11 patients completed the study. Two patients developed obstructive sleep apnea during sodium oxybate treatment (1 was the dropout) and were excluded from the per-protocol analysis (n = 10) but included in the intention-to-treat analysis (n = 12).
Interventions Nocturnal sodium oxybate and placebo taken at bedtime and 2.5 to 4.0 hours later with an individually titrated dose between 3.0 and 9.0 g per night for 6 weeks with a 2- to 4-week washout period interposed.
Main Outcomes and Measures Primary outcome measure was change of objective EDS as electrophysiologically measured by mean sleep latency in the Multiple Sleep Latency Test. Secondary outcome measures included change of subjective EDS (ESS), sleep quality (Parkinson Disease Sleep Scale–2), and objective variables of nighttime sleep (polysomnography).
Results Among 12 patients in the intention-to-treat population (10 men, 2 women; mean [SD] age, 62 [11.1] years; disease duration, 8.4 [4.6] years), sodium oxybate substantially improved EDS as measured objectively (mean sleep latency, +2.9 minutes; 95% CI, 2.1 to 3.8 minutes; P = .002) and subjectively (ESS score, −4.2 points ; 95% CI, −5.3 to −3.0 points; P = .001). Thereby, 8 (67%) patients exhibited an electrophysiologically defined positive treatment response. Moreover, sodium oxybate significantly enhanced subjective sleep quality and objectively measured slow-wave sleep duration (+72.7 minutes; 95% CI, 55.7 to 89.7 minutes; P < .001). Differences were more pronounced in the per-protocol analysis. Sodium oxybate was generally well tolerated under dose adjustments (no treatment-related dropouts), but it induced de novo obstructive sleep apnea in 2 patients and parasomnia in 1 patient, as detected by polysomnography, all of whom did not benefit from sodium oxybate treatment.
Conclusions and Relevance This study provides class I evidence for the efficacy of sodium oxybate in treating EDS and nocturnal sleep disturbance in patients with PD. Special monitoring with follow-up polysomnography is necessary to rule out treatment-related complications and larger follow-up trials with longer treatment durations are warranted for validation.
Trial Registration clinicaltrials.gov Identifier: NCT02111122
中文翻译:

氧化钠治疗帕金森氏病过多的白天嗜睡和睡眠障碍一项随机临床试验
重要性 觉醒障碍是帕金森病(PD)的常见且令人衰弱的非运动表现,但治疗选择很少。
目的探讨 夜间发作性发作性发作的一线治疗方法羟丁酸钠对PD患者白天过度嗜睡(EDS)和夜间睡眠受扰是否有效和安全。
设计,设置和参与者 在2015年1月9日至2017年2月24日期间进行的随机,双盲,安慰剂对照,交叉2a期研究。在瑞士苏黎世大学医院的睡眠实验室进行的单中心研究中,有18位患者在睡眠实验室筛查了PD和EDS(Epworth嗜睡量表[ESS]得分> 10)的患者。由于多导睡眠图诊断睡眠呼吸暂停而排除了5名患者,并且有1名患者撤回了同意书。因此,将12例患者随机分配至治疗顺序(羟丁酸钠,安慰剂或安慰剂,然后羟丁酸钠,比例1:1),并且由于冲洗期间发生了无关的不良事件而导致1例患者退出治疗后,有11例患者完成了治疗。学习。
干预 措施于睡前和2.5至4.0小时后服用夜间羟丁酸钠和安慰剂,分别以每晚3.0至9.0 g的剂量滴定,持续6周,并有2至4周的清除期。
主要结果和衡量指标 主要结果衡量指标是通过多次睡眠潜伏期测试中的平均睡眠潜伏期通过电生理学测量的客观EDS的变化。次要结果指标包括主观EDS(ESS),睡眠质量(帕金森病睡眠量表–2)和夜间睡眠的客观变量(多导睡眠图)的变化。
结果 在意向性治疗人群中的12例患者中(男10例,女2例;平均[SD]年龄62 [11.1]岁;病程8.4 [4.6]年),从客观上看,羟丁酸钠显着改善了EDS(平均睡眠潜伏期,+ 2.9分钟; 95%CI,2.1至3.8分钟;P = 0.002)和主观(ESS评分,-4.2点; 95%CI,-5.3至-3.0点;P = .001)。因此,有8名(67%)患者表现出电生理学上确定的积极治疗反应。此外,羟丁酸钠可显着提高主观睡眠质量并客观测量慢波睡眠时间(+72.7分钟; 95%CI,55.7至89.7分钟;P <.001)。在每个协议的分析中差异更加明显。经多导睡眠图检查可知,在剂量调整下,羟丁酸钠耐受性良好(无与治疗相关的辍学现象),但它诱发了2例患者从头开始阻塞性呼吸暂停,1例失眠,所有患者均未从羟丁酸钠治疗中受益。
结论与相关性 这项研究提供了I类证据,证明了羟丁酸钠治疗PD患者的EDS和夜间睡眠障碍的疗效。为了排除与治疗有关的并发症,必须进行随访多导睡眠监测仪的特殊监测,并且需要进行更长治疗时间的大型随访试验以进行验证。
试验注册 临床试验.gov标识符:NCT02111122











































 京公网安备 11010802027423号
京公网安备 11010802027423号