当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reduction of amide carbonyl group and formation of modified amino acids and dipeptides
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2015-04-10 03:22:22 M.L. Di Gioia , E.L. Belsito , A. Leggio , V. Leotta , E. Romio , C. Siciliano , A. Liguori
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2015-04-10 03:22:22 M.L. Di Gioia , E.L. Belsito , A. Leggio , V. Leotta , E. Romio , C. Siciliano , A. Liguori
|
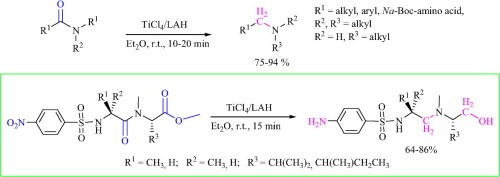
An expeditious, practical, and mild method for the reduction of amides to amines is reported. The procedure is based on the activation of amides with titanium tetrachloride followed by reduction with lithium aluminum hydride. The reducing system can be applied to the reduction of tertiary and secondary amides giving the corresponding amines in good yields. The protocol was also extended to the reduction of amides of Nα-protected amino acid and dipeptides. The corresponding 1,2 diamines and diaminoalcohols were produced in high yields and with retention of configuration at the chiral centers.
中文翻译:

还原酰胺羰基并形成修饰的氨基酸和二肽
报道了一种将酰胺还原为胺的快速,实用和温和的方法。该程序基于酰胺用四氯化钛活化,然后用氢化锂铝还原。该还原体系可用于叔酰胺和仲酰胺的还原,从而以良好的收率得到相应的胺。该方案还扩展到还原Nα-保护的氨基酸和二肽的酰胺。相应的1,2,二胺和二氨基醇以高收率生产,并且在手性中心保留构型。
更新日期:2015-04-10
中文翻译:

还原酰胺羰基并形成修饰的氨基酸和二肽
报道了一种将酰胺还原为胺的快速,实用和温和的方法。该程序基于酰胺用四氯化钛活化,然后用氢化锂铝还原。该还原体系可用于叔酰胺和仲酰胺的还原,从而以良好的收率得到相应的胺。该方案还扩展到还原Nα-保护的氨基酸和二肽的酰胺。相应的1,2,二胺和二氨基醇以高收率生产,并且在手性中心保留构型。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号