Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2017-10-31 , DOI: 10.1016/j.cej.2017.10.154 Rina Yamaguchi , Shunji Kurosu , Moe Suzuki , Yoshinori Kawase
|
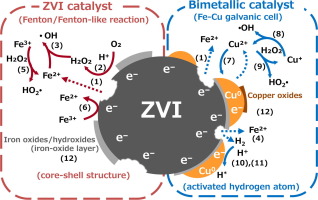
Zero-valent iron (ZVI) has been recognized as a heterogeneous Fenton/Fenton-like catalyst generating hydroxyl radical (OH radical). ZVI bimetallic catalysts modified by the deposition of transition metals on the ZVI surface have been proposed as alternatives to enhance the reactivity of ZVI catalyst. However, it is not clear from the literature whether OH radical is actively generated via the Fenton/Fenton-like reactions in ZVI bimetallic catalyst systems. To quantify the generation of OH radical by ZVI/Cu bimetallic catalysts, the amount of generated OH radical was measured under the oxic condition. Although the maximum amount of generated OH radical by ZVI/Cu bimetallic catalysts was obtained at pH 3 being an optimal pH for the Fenton reaction, OH radical generation by ZVI/Cu bimetallic catalysts was considerably restricted by the inadequate in-situ generation of H2O2 and the generation of OH radical was inhibited due to Cu deposition on ZVI surface. While the deposition of Cu on the ZVI surface enhanced the removal of Orange II via the facilitation of reductive degradation and the increase in adsorption capability on the surface of ZVI/Cu bimetallic catalysts, it could not stimulate the OH radical generation or the oxidative reactivity of heterogeneous Fenton/Fenton-like catalyst. The present study confirmed that the passivation of ZVI surface accelerated by the deposition of Cu on ZVI surface inhibited the OH radical generation. The kinetic models for OH radical generation by ZVI/Cu bimetallic catalysts were developed by considering the linkage of OH radical generation with eluted Fe and Cu ions. The model predictions could simulate the experimental results reasonably.
中文翻译:

零价铁/铜(ZVI / Cu)双金属催化剂在废水处理中产生的羟基自由基:在有氧条件下通过原位形成的Fenton试剂进行非均相Fenton /类Fenton反应
零价铁(ZVI)被认为是生成羟基自由基(OH自由基)的非均相芬顿/类芬顿催化剂。已经提出通过在ZVI表面上沉积过渡金属而改性的ZVI双金属催化剂作为增强ZVI催化剂的反应性的替代物。然而,从文献中尚不清楚在ZVI双金属催化剂体系中是否经由Fenton /类Fenton反应主动地产生OH自由基。为了量化ZVI / Cu双金属催化剂生成的OH自由基,在有氧条件下测量了生成的OH自由基的量。尽管ZVI / Cu双金属催化剂产生的最大OH自由基量是在Fenton反应的最佳pH值为3的情况下获得的,2 O 2由于在ZVI表面沉积了铜,抑制了OH自由基的产生。虽然ZVI表面上的Cu沉积通过促进还原降解和ZVI / Cu双金属催化剂表面吸附能力的提高而增强了Orange II的去除,但它不能刺激OH自由基的产生或氧化活性。非均相的Fenton /类Fenton催化剂。本研究证实,通过在ZVI表面沉积Cu加速了ZVI表面的钝化,抑制了OH自由基的产生。通过考虑OH自由基生成与洗脱的Fe和Cu离子的联系,建立了ZVI / Cu双金属催化剂生成OH自由基的动力学模型。模型预测可以合理地模拟实验结果。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号