当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of 3,5-Diphenyl-4-methyl-1,3-oxazolidin-2-ones as Novel, Potent, and Orally Available Δ-5 Desaturase (D5D) Inhibitors
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2017-10-31 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01210 Jun Fujimoto 1 , Rei Okamoto 1 , Naoyoshi Noguchi 1 , Ryoma Hara 1 , Shinichi Masada 1 , Tetsuji Kawamoto 1 , Hiroki Nagase 1 , Yumiko Okano Tamura 1 , Mitsuaki Imanishi 1 , Shuichi Takagahara 1 , Kazuki Kubo 1 , Kimio Tohyama 1 , Koichi Iida 1 , Tomohiro Andou 1 , Ikuo Miyahisa 1 , Junji Matsui 1 , Ryouta Hayashi 1 , Tsuyoshi Maekawa 1 , Nobuyuki Matsunaga 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2017-10-31 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01210 Jun Fujimoto 1 , Rei Okamoto 1 , Naoyoshi Noguchi 1 , Ryoma Hara 1 , Shinichi Masada 1 , Tetsuji Kawamoto 1 , Hiroki Nagase 1 , Yumiko Okano Tamura 1 , Mitsuaki Imanishi 1 , Shuichi Takagahara 1 , Kazuki Kubo 1 , Kimio Tohyama 1 , Koichi Iida 1 , Tomohiro Andou 1 , Ikuo Miyahisa 1 , Junji Matsui 1 , Ryouta Hayashi 1 , Tsuyoshi Maekawa 1 , Nobuyuki Matsunaga 1
Affiliation
|
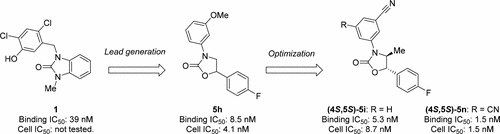
The discovery and optimization of Δ-5 desaturase (D5D) inhibitors are described. Investigation of the 1,3-oxazolidin-2-one scaffold was inspired by a pharmacophore model constructed from the common features of several hit compounds, resulting in the identification of 3,5-diphenyl-1,3-oxazolidin-2-one 5h as a novel lead showing potent in vitro activity. Subsequent optimization focused on the modification of two metabolic sites, which provided (4S,5S)-5i, a derivative with improved metabolic stability. Moreover, adding a substituent into the upper phenyl moiety further enhanced the intrinsic activity, which led to the discovery of 5-[(4S,5S)-5-(4fluorophenyl)-4-methyl-2-oxo-1,3-oxazolidin-3-yl]benzene-1,3-dicarbonitrile (4S,5S)-5n, endowed with excellent D5D binding affinity, cellular activity, and high oral bioavailability in a mouse. It exhibited robust in vivo hepatic arachidonic acid/dihomo-γ-linolenic acid ratio reduction (a target engagement marker) in an atherosclerosis mouse model. Finally, an asymmetric synthetic procedure for this compound was established.
中文翻译:

3,5-二苯基-4-甲基-1,3-恶唑烷-2-酮作为新型,有效和口服可用的Δ-5去饱和酶(D5D)抑制剂的发现
描述并优化了Δ-5去饱和酶(D5D)抑制剂。1,3-恶唑烷-2-酮骨架的研究受到药效团模型的启发,该药效团模型是由几种命中化合物的共同特征构建而成的,从而可在5h内鉴定出3,5-二苯基-1,3-恶唑烷-2-酮作为一种新型的铅,显示出强大的体外活性。随后的优化集中在两个代谢位点的修饰上,这提供了(4 S,5 S)-5i,这是一种具有改善的代谢稳定性的衍生物。此外,在上部苯基部分添加取代基进一步增强了内在活性,这导致了5-[(4 S,5 S)-5-(4氟苯基)-4-甲基-2-氧代-1,3-恶唑烷-3-基]苯-1,3-二碳腈(4 S,5 S)-5n,具有出色的D5D结合亲和力,细胞活性和小鼠的高口服生物利用度。在动脉粥样硬化小鼠模型中,它表现出强劲的体内肝花生四烯酸/二高-γ-亚麻酸比率降低(目标参与标记)。最后,建立了该化合物的不对称合成方法。
更新日期:2017-11-01
中文翻译:

3,5-二苯基-4-甲基-1,3-恶唑烷-2-酮作为新型,有效和口服可用的Δ-5去饱和酶(D5D)抑制剂的发现
描述并优化了Δ-5去饱和酶(D5D)抑制剂。1,3-恶唑烷-2-酮骨架的研究受到药效团模型的启发,该药效团模型是由几种命中化合物的共同特征构建而成的,从而可在5h内鉴定出3,5-二苯基-1,3-恶唑烷-2-酮作为一种新型的铅,显示出强大的体外活性。随后的优化集中在两个代谢位点的修饰上,这提供了(4 S,5 S)-5i,这是一种具有改善的代谢稳定性的衍生物。此外,在上部苯基部分添加取代基进一步增强了内在活性,这导致了5-[(4 S,5 S)-5-(4氟苯基)-4-甲基-2-氧代-1,3-恶唑烷-3-基]苯-1,3-二碳腈(4 S,5 S)-5n,具有出色的D5D结合亲和力,细胞活性和小鼠的高口服生物利用度。在动脉粥样硬化小鼠模型中,它表现出强劲的体内肝花生四烯酸/二高-γ-亚麻酸比率降低(目标参与标记)。最后,建立了该化合物的不对称合成方法。































 京公网安备 11010802027423号
京公网安备 11010802027423号