当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Docking-based design and synthesis of galantamine–camphane hybrids as inhibitors of acetylcholinesterase
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2017-05-12 07:16:10 , DOI: 10.1111/cbdd.12991 Georgi Stavrakov 1 , Irena Philipova 2 , Dimitrina Zheleva-Dimitrova 1 , Iva Valkova 1 , Evdokiya Salamanova 3 , Spiro Konstantinov 1 , Irini Doytchinova 1
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2017-05-12 07:16:10 , DOI: 10.1111/cbdd.12991 Georgi Stavrakov 1 , Irena Philipova 2 , Dimitrina Zheleva-Dimitrova 1 , Iva Valkova 1 , Evdokiya Salamanova 3 , Spiro Konstantinov 1 , Irini Doytchinova 1
Affiliation
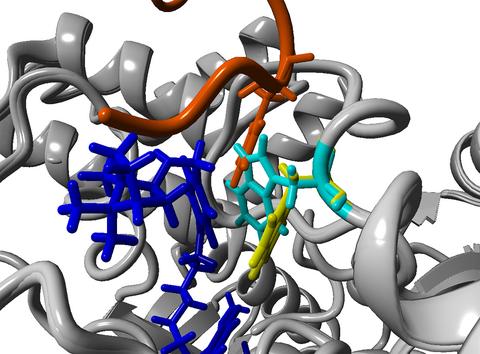
|
The galantamine fragment of the galantamine–camphane hybrids bind the catalytic site of acetylcholinesterase, while the camphane fragment fits in the peripheral anionic site, where the Ω-loop of amyloid beta peptide binds to the enzyme. The inhibitor “closes” the binding site by causing a shift of Trp286 and prevents the amyloid beta peptide from binding.
中文翻译:

基于对接的加兰他敏-樟脑杂种作为乙酰胆碱酯酶抑制剂的设计和合成
加兰他敏-樟脑杂化物的加兰他敏片段结合了乙酰胆碱酯酶的催化位点,而樟脑片段适合于周围的阴离子位点,淀粉样β肽的Ω环与酶结合。抑制剂通过引起Trp286转移来“封闭”结合位点,并阻止淀粉样β肽结合。
更新日期:2017-11-01
中文翻译:

基于对接的加兰他敏-樟脑杂种作为乙酰胆碱酯酶抑制剂的设计和合成
加兰他敏-樟脑杂化物的加兰他敏片段结合了乙酰胆碱酯酶的催化位点,而樟脑片段适合于周围的阴离子位点,淀粉样β肽的Ω环与酶结合。抑制剂通过引起Trp286转移来“封闭”结合位点,并阻止淀粉样β肽结合。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号