当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An expedient synthesis of N-(1-(5-mercapto-4-((substituted benzylidene)amino)-4H-1,2,4-triazol-3-yl)-2-phenylethyl)benzamides as jack bean urease inhibitors and free radical scavengers: Kinetic mechanism and molecular docking studies
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2017-06-19 06:11:02 , DOI: 10.1111/cbdd.12998 Aamer Saeed 1 , Fayaz Ali Larik 1 , Pervaiz Ali Channar 1 , Haroon Mehfooz 1 , Mohammad Haseeb Ashraf 1 , Qamar Abbas 2 , Mubashir Hassan 2 , Sung-Yum Seo 2
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2017-06-19 06:11:02 , DOI: 10.1111/cbdd.12998 Aamer Saeed 1 , Fayaz Ali Larik 1 , Pervaiz Ali Channar 1 , Haroon Mehfooz 1 , Mohammad Haseeb Ashraf 1 , Qamar Abbas 2 , Mubashir Hassan 2 , Sung-Yum Seo 2
Affiliation
|
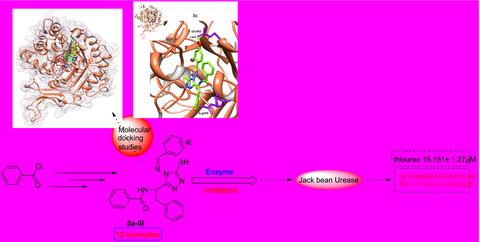
Synthesis of N-(1-(5-mercapto-4-((substituted benzylidene) amino)-4H-1, 2, 4-triazol-3-yl)-2-phenylethyl) benzamides as jack bean urease and free radical scavenger inhibitors. Kinetic mechanism revealed that most potent derivative 5j inhibits enzyme by non-competitive mechanism. The binding affinity of the molecules was evaluated using molecular docking studies. Based on the results of enzyme inhibition activities and molecular docking, it was inferred that these molecules can serve as template for the drug designing and discovery. Further structural modifications to most potent derivatives can lead to the designing of efficient enzyme inhibitors.
中文翻译:

方便合成的N-(1-(5-巯基-4-((取代的亚苄基)氨基)-4H-1,2,4-三唑-3-基)-2-苯基乙基)苯甲酰胺作为杰克豆脲酶抑制剂和自由基清除剂:动力学机理和分子对接研究
的合成ñ - (1-(5-巯基-4 - ((取代亚苄基)氨基)-4- ħ -1,2,4-三唑-3-基)-2-苯基乙基)苯甲酰胺如刀豆脲酶和自由基清除剂抑制剂。动力学机制表明,最有效的衍生物5j通过非竞争性机制抑制酶。使用分子对接研究评估了分子的结合亲和力。根据酶抑制活性和分子对接的结果,推断这些分子可以作为药物设计和发现的模板。对大多数有效衍生物的进一步结构修饰可导致设计有效的酶抑制剂。
更新日期:2017-11-01
中文翻译:

方便合成的N-(1-(5-巯基-4-((取代的亚苄基)氨基)-4H-1,2,4-三唑-3-基)-2-苯基乙基)苯甲酰胺作为杰克豆脲酶抑制剂和自由基清除剂:动力学机理和分子对接研究
的合成ñ - (1-(5-巯基-4 - ((取代亚苄基)氨基)-4- ħ -1,2,4-三唑-3-基)-2-苯基乙基)苯甲酰胺如刀豆脲酶和自由基清除剂抑制剂。动力学机制表明,最有效的衍生物5j通过非竞争性机制抑制酶。使用分子对接研究评估了分子的结合亲和力。根据酶抑制活性和分子对接的结果,推断这些分子可以作为药物设计和发现的模板。对大多数有效衍生物的进一步结构修饰可导致设计有效的酶抑制剂。






























 京公网安备 11010802027423号
京公网安备 11010802027423号