当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Robust, Chiral, and Porous BINAP-Based Metal–Organic Frameworks for Highly Enantioselective Cyclization Reactions
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2015-09-15 , DOI: 10.1021/jacs.5b09225 Takahiro Sawano 1 , Nathan C. Thacker 1 , Zekai Lin 1 , Alexandra R. McIsaac 1 , Wenbin Lin 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2015-09-15 , DOI: 10.1021/jacs.5b09225 Takahiro Sawano 1 , Nathan C. Thacker 1 , Zekai Lin 1 , Alexandra R. McIsaac 1 , Wenbin Lin 1
Affiliation
|
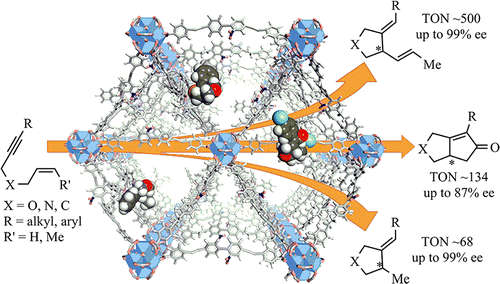
We report here the design of BINAP-based metal-organic frameworks and their postsynthetic metalation with Rh complexes to afford highly active and enantioselective single-site solid catalysts for the asymmetric cyclization reactions of 1,6-enynes. Robust, chiral, and porous Zr-MOFs of UiO topology, BINAP-MOF (I) or BINAP-dMOF (II), were prepared using purely BINAP-derived dicarboxylate linkers or by mixing BINAP-derived linkers with unfunctionalized dicarboxylate linkers, respectively. Upon metalation with Rh(nbd)2BF4 and [Rh(nbd)Cl]2/AgSbF6, the MOF precatalysts I·Rh(BF4) and I·Rh(SbF6) efficiently catalyzed highly enantioselective (up to 99% ee) reductive cyclization and Alder-ene cycloisomerization of 1,6-enynes, respectively. I·Rh catalysts afforded cyclization products at comparable enantiomeric excesses (ee's) and 4-7 times higher catalytic activity than the homogeneous controls, likely a result of catalytic site isolation in the MOF which prevents bimolecular catalyst deactivation pathways. However, I·Rh is inactive in the more sterically encumbered Pauson-Khand reactions between 1,6-enynes and carbon monoxide. In contrast, with a more open structure, Rh-functionalized BINAP-dMOF, II·Rh, effectively catalyzed Pauson-Khand cyclization reactions between 1,6-enynes and carbon monoxide at 10 times higher activity than the homogeneous control. II·Rh was readily recovered and used three times in Pauson-Khand cyclization reactions without deterioration of yields or ee's. Our work has expanded the scope of MOF-catalyzed asymmetric reactions and showed that the mixed linker strategy can effectively enlarge the open space around the catalytic active site to accommodate highly sterically demanding polycyclic metallocycle transition states/intermediates in asymmetric intramolecular cyclization reactions.
中文翻译:

用于高度对映选择性环化反应的坚固、手性和多孔 BINAP 基金属-有机骨架
我们在此报告了基于 BINAP 的金属有机骨架的设计及其与 Rh 配合物的合成后金属化,为 1,6-烯炔的不对称环化反应提供高活性和对映选择性的单中心固体催化剂。UiO 拓扑结构的坚固、手性和多孔 Zr-MOF,BINAP-MOF (I) 或 BINAP-dMOF (II),分别使用纯 BINAP 衍生的二羧酸酯接头或通过将 BINAP 衍生的接头与未官能化的二羧酸酯接头混合来制备。在用 Rh(nbd)2BF4 和 [Rh(nbd)Cl]2/AgSbF6 金属化后,MOF 预催化剂 I·Rh(BF4) 和 I·Rh(SbF6) 有效地催化了高度对映选择性(高达 99% ee)的还原环化和1,6-烯炔的阿尔德烯环异构化,分别。I·Rh 催化剂在相当的对映体过量(ee' s) 和比均相对照高 4-7 倍的催化活性,这可能是 MOF 中催化位点隔离的结果,这防止了双分子催化剂失活途径。然而,I·Rh 在 1,6-烯炔和一氧化碳之间空间受限的 Pauson-Khand 反应中是不活跃的。相比之下,Rh 功能化的 BINAP-dMOF II·Rh 具有更开放的结构,可有效催化 1,6-烯炔和一氧化碳之间的 Pauson-Khand 环化反应,活性比均相对照高 10 倍。II·Rh 很容易回收并在 Pauson-Khand 环化反应中使用 3 次,而不会降低收率或 ee's。
更新日期:2015-09-15
中文翻译:

用于高度对映选择性环化反应的坚固、手性和多孔 BINAP 基金属-有机骨架
我们在此报告了基于 BINAP 的金属有机骨架的设计及其与 Rh 配合物的合成后金属化,为 1,6-烯炔的不对称环化反应提供高活性和对映选择性的单中心固体催化剂。UiO 拓扑结构的坚固、手性和多孔 Zr-MOF,BINAP-MOF (I) 或 BINAP-dMOF (II),分别使用纯 BINAP 衍生的二羧酸酯接头或通过将 BINAP 衍生的接头与未官能化的二羧酸酯接头混合来制备。在用 Rh(nbd)2BF4 和 [Rh(nbd)Cl]2/AgSbF6 金属化后,MOF 预催化剂 I·Rh(BF4) 和 I·Rh(SbF6) 有效地催化了高度对映选择性(高达 99% ee)的还原环化和1,6-烯炔的阿尔德烯环异构化,分别。I·Rh 催化剂在相当的对映体过量(ee' s) 和比均相对照高 4-7 倍的催化活性,这可能是 MOF 中催化位点隔离的结果,这防止了双分子催化剂失活途径。然而,I·Rh 在 1,6-烯炔和一氧化碳之间空间受限的 Pauson-Khand 反应中是不活跃的。相比之下,Rh 功能化的 BINAP-dMOF II·Rh 具有更开放的结构,可有效催化 1,6-烯炔和一氧化碳之间的 Pauson-Khand 环化反应,活性比均相对照高 10 倍。II·Rh 很容易回收并在 Pauson-Khand 环化反应中使用 3 次,而不会降低收率或 ee's。













































 京公网安备 11010802027423号
京公网安备 11010802027423号