当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Following the Molecular Mechanism of Decarbonylation of Unsaturated Cyclic Ketones Using Bonding Evolution Theory Coupled with NCI Analysis
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-11-01 00:00:00 , DOI: 10.1021/acs.jpca.7b08503
Ehsan Zahedi 1 , Samaneh Shaabani 1 , Abolfazl Shiroudi 2
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-11-01 00:00:00 , DOI: 10.1021/acs.jpca.7b08503
Ehsan Zahedi 1 , Samaneh Shaabani 1 , Abolfazl Shiroudi 2
Affiliation
|
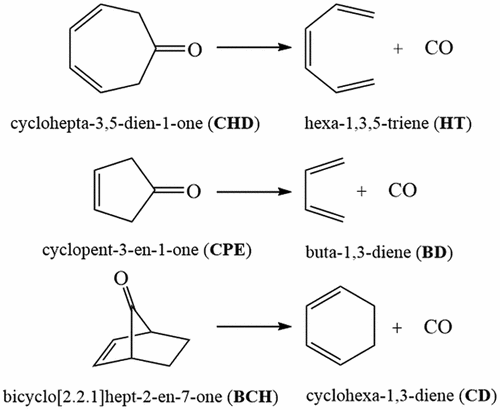
The synergetic use of bonding evolution theory (BET) and noncovalent interaction (NCI) analysis allows to obtain new insight into the bond breaking/forming processes and electron redistribution along the reaction path to understand the molecular mechanism of a reaction and recognize regions of strong and weak electron pairing. This viewpoint has been considered for cheletropic extrusion of CO from unsaturated cyclic ketones cyclohepta-3,5-dien-1-one CHD, cyclopent–3-en-1-one CPE, and bicyclo[2.2.1]hept-2-en-7-one BCH by using hybrid functional MPWB1K in conjugation with aug-cc-pVTZ basis set. Decarbonylation of CHD, CPE, and BCH are nonpolar cyclo-elimination reactions that are characterized by the sequence of turning points (TPs) as CHD, 1–11-C[CC]C†C†FFFTSC†C†C†–0:HT + CO; CPE, 1–8-CC[C†C†F†][FF][FF]FTS[C†C†]–0:BD + CO; and BCH, 1–8-CC[C†C†]F[FF]FTS[C†C†]–0:CD + CO. Breaking of C–C bond between the terminal carbon atoms of diene/triene framework and carbon atom of CO fragment starts at a distance of ca. 1.9–2.0 Å in the vicinity of the transition structure where the transition states are not reached yet. NCI analysis explains that the noncovalent interactions between two fragments appeared after the breaking of C–C bonds.
中文翻译:

结合键合演化理论和NCI分析的不饱和环酮脱羰分子机理
通过结合使用键演化理论(BET)和非共价相互作用(NCI)分析,可以对键断裂/形成过程和电子沿着反应路径的重新分布有了新的认识,从而了解反应的分子机理并识别强和弱的区域。电子弱配对。考虑从非饱和环状酮的偏向挤出CO的观点,即环庚3,5- dien-1-one CHD,环戊3烯-1-en CPE和双环[2.2.1]庚2烯通过使用混合功能MPWB1K与aug-cc-pVTZ基集结合使用-7位BCH。CHD,CPE和BCH的脱羰作用是非极性环消除反应,其特征在于转折点(TPs)的顺序为CHD,1–11-C [CC] C † C † FFF TS C † C † C † –0:HT + CO;CPE,1–8-CC [C † C † F † ] [FF] [FF] F TS [C † C † ] –0:BD + CO;和BCH,1–8-CC [C † C † ] F [FF] F TS [C † C † ] –0:CD二烯/三烯骨架的末端碳原子与CO片段碳原子之间的C–C键断裂始于大约距离。在尚未达到过渡状态的过渡结构附近为1.9–2.0Å。NCI分析说明,两个片段之间的非共价相互作用是在C–C键断裂后出现的。
更新日期:2017-11-01
中文翻译:

结合键合演化理论和NCI分析的不饱和环酮脱羰分子机理
通过结合使用键演化理论(BET)和非共价相互作用(NCI)分析,可以对键断裂/形成过程和电子沿着反应路径的重新分布有了新的认识,从而了解反应的分子机理并识别强和弱的区域。电子弱配对。考虑从非饱和环状酮的偏向挤出CO的观点,即环庚3,5- dien-1-one CHD,环戊3烯-1-en CPE和双环[2.2.1]庚2烯通过使用混合功能MPWB1K与aug-cc-pVTZ基集结合使用-7位BCH。CHD,CPE和BCH的脱羰作用是非极性环消除反应,其特征在于转折点(TPs)的顺序为CHD,1–11-C [CC] C † C † FFF TS C † C † C † –0:HT + CO;CPE,1–8-CC [C † C † F † ] [FF] [FF] F TS [C † C † ] –0:BD + CO;和BCH,1–8-CC [C † C † ] F [FF] F TS [C † C † ] –0:CD二烯/三烯骨架的末端碳原子与CO片段碳原子之间的C–C键断裂始于大约距离。在尚未达到过渡状态的过渡结构附近为1.9–2.0Å。NCI分析说明,两个片段之间的非共价相互作用是在C–C键断裂后出现的。







































 京公网安备 11010802027423号
京公网安备 11010802027423号