当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Probing ligand exchange in the P450 enzyme CYP121 from Mycobacterium tuberculosis: Dynamic equilibrium of the distal heme ligand as a function of pH and temperature
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-11-20 , DOI: 10.1021/jacs.7b08911 Andrew J Fielding 1 , Kednerlin Dornevil 1 , Li Ma 1 , Ian Davis 1 , Aimin Liu 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-11-20 , DOI: 10.1021/jacs.7b08911 Andrew J Fielding 1 , Kednerlin Dornevil 1 , Li Ma 1 , Ian Davis 1 , Aimin Liu 1
Affiliation
|
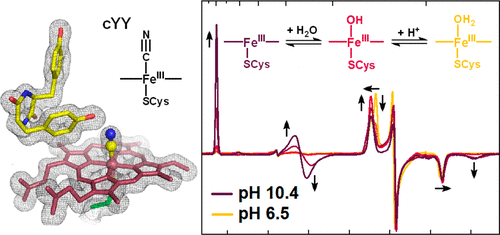
CYP121 is a cytochrome P450 enzyme from Mycobacterium tuberculosis that catalyzes the formation of a C-C bond between the aromatic groups of its cyclodityrosine substrate (cYY). The crystal structure of CYP121 in complex with cYY reveals that the solvent-derived ligand remains bound to the ferric ion in the enzyme-substrate complex. Whereas in the generally accepted P450 mechanism, binding of the primary substrate in the active-site triggers the release of the solvent-derived ligand, priming the metal center for reduction and subsequent O2 binding. Here we employed sodium cyanide to probe the metal-ligand exchange of the enzyme and the enzyme-substrate complex. The cyano adducts were characterized by UV-vis, EPR, and ENDOR spectroscopies and X-ray crystallography. A 100-fold increase in the affinity of cyanide binding to the enzyme-substrate complex over the ligand-free enzyme was observed. The crystal structure of the [CYP121(cYY)CN] ternary complex showed a rearrangement of the substrate in the active-site, when compared to the structure of the binary [CYP121(cYY)] complex. Transient kinetic studies showed that cYY binding resulted in a lower second-order rate constant (kon (CN)) but a much more stable cyanide adduct with 3 orders of magnitude slower koff (CN) rate. A dynamic equilibrium between multiple high- and low-spin species for both the enzyme and enzyme-substrate complex was also observed, which is sensitive to changes in both pH and temperature. Our data reveal the chemical and physical properties of the solvent-derived ligand of the enzyme, which will help to understand the initial steps of the catalytic mechanism.
中文翻译:

探测结核分枝杆菌 P450 酶 CYP121 中的配体交换:远端血红素配体作为 pH 和温度函数的动态平衡
CYP121 是一种来自结核分枝杆菌的细胞色素 P450 酶,可催化其环二酪氨酸底物 (cYY) 的芳香族基团之间形成 CC 键。 CYP121 与 cYY 复合物的晶体结构表明,溶剂衍生的配体仍然与酶-底物复合物中的铁离子结合。而在普遍接受的 P450 机制中,活性位点中主要底物的结合会触发溶剂衍生配体的释放,从而启动金属中心进行还原和随后的 O2 结合。在这里,我们使用氰化钠来探测酶和酶-底物复合物的金属-配体交换。通过 UV-vis、EPR、ENDOR 光谱和 X 射线晶体学对氰基加合物进行了表征。与无配体的酶相比,氰化物与酶-底物复合物的结合亲和力增加了 100 倍。与二元[CYP121(cYY)]复合物的结构相比,[CYP121(cYY)CN]三元复合物的晶体结构显示活性位点中底物的重排。瞬态动力学研究表明,cYY 结合导致较低的二阶速率常数 (kon (CN)),但氰化物加合物更加稳定,koff (CN) 速率减慢 3 个数量级。还观察到酶和酶-底物复合物的多个高自旋和低自旋物种之间的动态平衡,该平衡对 pH 和温度的变化都很敏感。我们的数据揭示了酶的溶剂衍生配体的化学和物理性质,这将有助于理解催化机制的初始步骤。
更新日期:2017-11-20
中文翻译:

探测结核分枝杆菌 P450 酶 CYP121 中的配体交换:远端血红素配体作为 pH 和温度函数的动态平衡
CYP121 是一种来自结核分枝杆菌的细胞色素 P450 酶,可催化其环二酪氨酸底物 (cYY) 的芳香族基团之间形成 CC 键。 CYP121 与 cYY 复合物的晶体结构表明,溶剂衍生的配体仍然与酶-底物复合物中的铁离子结合。而在普遍接受的 P450 机制中,活性位点中主要底物的结合会触发溶剂衍生配体的释放,从而启动金属中心进行还原和随后的 O2 结合。在这里,我们使用氰化钠来探测酶和酶-底物复合物的金属-配体交换。通过 UV-vis、EPR、ENDOR 光谱和 X 射线晶体学对氰基加合物进行了表征。与无配体的酶相比,氰化物与酶-底物复合物的结合亲和力增加了 100 倍。与二元[CYP121(cYY)]复合物的结构相比,[CYP121(cYY)CN]三元复合物的晶体结构显示活性位点中底物的重排。瞬态动力学研究表明,cYY 结合导致较低的二阶速率常数 (kon (CN)),但氰化物加合物更加稳定,koff (CN) 速率减慢 3 个数量级。还观察到酶和酶-底物复合物的多个高自旋和低自旋物种之间的动态平衡,该平衡对 pH 和温度的变化都很敏感。我们的数据揭示了酶的溶剂衍生配体的化学和物理性质,这将有助于理解催化机制的初始步骤。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号