当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Speciated Monitoring of Gas-Phase Organic Peroxy Radicals by Chemical Ionization Mass Spectrometry: Cross-Reactions between CH3O2, CH3(CO)O2, (CH3)3CO2, and c-C6H11O2
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-10-30 00:00:00 , DOI: 10.1021/acs.jpca.7b06456 Barbara Nozière 1 , David R. Hanson 2
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-10-30 00:00:00 , DOI: 10.1021/acs.jpca.7b06456 Barbara Nozière 1 , David R. Hanson 2
Affiliation
|
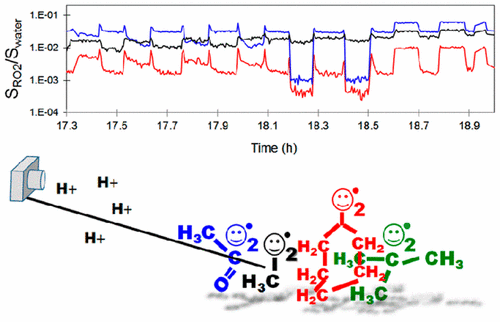
Organic peroxy radicals (“RO2”, with R organic) are key intermediates in most oxygen-rich systems, where organic compounds are oxidized (natural environment, flames, combustion engines, living organisms, etc). But, until recently, techniques able to monitor simultaneously and distinguish between RO2 species (“speciated” detection) have been scarce, which has limited the understanding of complex systems containing these radicals. Mass spectrometry using proton transfer ionization has been shown previously to detect individual gas-phase RO2 separately. In this work, we illustrate its ability to speciate and monitor several RO2 simultaneously by investigating reactions involving CH3O2, CH3C(O)O2, c-C6H11O2, and (CH3)3CO2. The detection sensitivity of each of these radicals was estimated by titration with NO to between 50 and 1000 Hz/ppb, with a factor from 3 to 5 of uncertainties, mostly due to the uncertainties in knowing the amounts of added NO. With this, the RO2 concentration in the reactor was estimated between 1 × 1010 and 1 × 1012 molecules cm–3. When adding a second radical species to the reactor, the kinetics of the cross-reaction could be studied directly from the decay of the first radical. The time-evolution of two and sometimes three different RO2 was followed simultaneously, as the CH3O2 produced in further reaction steps was also detected in some systems. The rate coefficients obtained are (in molecule–1 cm3 s–1): kCH3O2+CH3C(O)O2 = 1.2 × 10–11, kCH3O2+t-butylO2 = 3.0 × 10–15, kc-hexylO2+CH3O2 = 1.2 × 10–13, kt-butylO2+CH3C(O)O2 = 3.7 × 10–14, and kc-hexylO2+t-butylO2 = 1.5 × 10–15. In spite of their good comparison with the literature and good reproducibility, large uncertainties (×5/5) are recommended on these results because of those in the detection sensitivities. This work is a first illustration of the potential applications of this technique for the investigation of organic radicals in laboratory and in more complex systems.
中文翻译:

通过化学电离质谱法专门监测气相有机过氧自由基:CH 3 O 2,CH 3(CO)O 2,(CH 3)3 CO 2和cC 6 H 11 O 2之间的交叉反应
有机过氧自由基(“ RO 2 ”,R为有机物)是大多数富含氧气的系统中的关键中间体,在这些系统中,有机化合物被氧化(自然环境,火焰,内燃机,活生物体等)。但是,直到最近,还缺乏能够同时监视和区分RO 2种类(“指定”检测)的技术,这限制了对包含这些自由基的复杂系统的理解。先前已经显示了使用质子转移电离的质谱法来分别检测单独的气相RO 2。在这项工作中,我们通过研究涉及CH 3 O 2的反应来说明其同时鉴定和监测多个RO 2的能力。,CH 3 C(O)O 2,cC 6 H 11 O 2和(CH 3)3 CO 2。这些自由基中每一个的检测灵敏度是通过用NO滴定至50至1000 Hz / ppb之间来估计的,不确定度为3-5,这主要是由于知道添加的NO量的不确定性所致。据此,估计反应器中的RO 2浓度在1×10 10和1×10 12分子cm –3之间。当向反应器中添加第二种自由基时,可以直接从第一个自由基的衰变中研究交叉反应的动力学。同时跟踪两个,有时三个不同的RO 2的时间演化,因为在某些系统中还检测到在进一步的反应步骤中生成的CH 3 O 2。获得的速率系数为(在分子中–1 cm 3 s –1):k CH3O2 + CH3C(O)O2 = 1.2×10 –11,k CH3O2 +叔丁基O2 = 3.0×10 –15,k c-己基O2 + CH3O2 = 1.2×10 –13,k叔丁基O2 + CH3C(O)O2 = 3.7×10 –14,k c-己基O2 +叔丁基O2 = 1.5×10 –15。尽管与文献进行了很好的比较并且具有良好的可重复性,但是由于检测灵敏度的原因,建议对这些结果进行较大的不确定性(×5/5)。这项工作是对该技术在实验室和更复杂的系统中研究有机自由基的潜在应用的首次说明。
更新日期:2017-10-30
中文翻译:

通过化学电离质谱法专门监测气相有机过氧自由基:CH 3 O 2,CH 3(CO)O 2,(CH 3)3 CO 2和cC 6 H 11 O 2之间的交叉反应
有机过氧自由基(“ RO 2 ”,R为有机物)是大多数富含氧气的系统中的关键中间体,在这些系统中,有机化合物被氧化(自然环境,火焰,内燃机,活生物体等)。但是,直到最近,还缺乏能够同时监视和区分RO 2种类(“指定”检测)的技术,这限制了对包含这些自由基的复杂系统的理解。先前已经显示了使用质子转移电离的质谱法来分别检测单独的气相RO 2。在这项工作中,我们通过研究涉及CH 3 O 2的反应来说明其同时鉴定和监测多个RO 2的能力。,CH 3 C(O)O 2,cC 6 H 11 O 2和(CH 3)3 CO 2。这些自由基中每一个的检测灵敏度是通过用NO滴定至50至1000 Hz / ppb之间来估计的,不确定度为3-5,这主要是由于知道添加的NO量的不确定性所致。据此,估计反应器中的RO 2浓度在1×10 10和1×10 12分子cm –3之间。当向反应器中添加第二种自由基时,可以直接从第一个自由基的衰变中研究交叉反应的动力学。同时跟踪两个,有时三个不同的RO 2的时间演化,因为在某些系统中还检测到在进一步的反应步骤中生成的CH 3 O 2。获得的速率系数为(在分子中–1 cm 3 s –1):k CH3O2 + CH3C(O)O2 = 1.2×10 –11,k CH3O2 +叔丁基O2 = 3.0×10 –15,k c-己基O2 + CH3O2 = 1.2×10 –13,k叔丁基O2 + CH3C(O)O2 = 3.7×10 –14,k c-己基O2 +叔丁基O2 = 1.5×10 –15。尽管与文献进行了很好的比较并且具有良好的可重复性,但是由于检测灵敏度的原因,建议对这些结果进行较大的不确定性(×5/5)。这项工作是对该技术在实验室和更复杂的系统中研究有机自由基的潜在应用的首次说明。































 京公网安备 11010802027423号
京公网安备 11010802027423号