当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
How Does the C–Halogen Bond Break in the Photosubstitution Reaction of 3-Fluorobenzophenone in Acidic Aqueous Solutions?
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2015-09-14 00:00:00 , DOI: 10.1021/acs.joc.5b01308 Jinqing Huang 1 , Jiani Ma 1, 2 , Mingde Li 1 , Mingyue Liu 1 , Xiting Zhang 1 , David Lee Phillips 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2015-09-14 00:00:00 , DOI: 10.1021/acs.joc.5b01308 Jinqing Huang 1 , Jiani Ma 1, 2 , Mingde Li 1 , Mingyue Liu 1 , Xiting Zhang 1 , David Lee Phillips 1
Affiliation
|
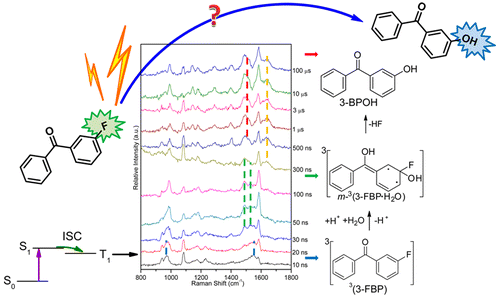
The efficient photosubstitution reaction of m-fluorobenzophenone and the related photohydration reactions were systematically investigated in acidic aqueous solutions. The mechanisms and intermediates were directly characterized by femtosecond transient absorption spectroscopy and nanosecond time-resolved resonance Raman spectroscopy, which is supported by density functional theory calculations. This photosubstitution was found to be a two-step process, based on the observation of a meta-hydration intermediate. The protonation of the ketone was confirmed as a crucial precursor step for further photochemical reactions as indicated by the observation of the absorption spectrum of an excited triplet protonated species. More interestingly, the efficient photosubstitution reaction could selectively occur under specific conditions. Control experiments on a series of halogen-substituted benzophenones were conducted to study the influence of the solution acidity, substituent positions, and the kind of substituted halogens on the efficiency in forming the corresponding hydroxyl photosubstitution product. Some practical conditions in predicting the efficiency of the photosubstitution reaction of interest are summarized, and they were successfully used to predict when the photosubstitution reaction takes place for some other halogen-substituted benzophenone derivatives. The driving force of this photosubstitution reaction may provide insights into several possible applications which are also briefly discussed.
中文翻译:

在酸性水溶液中3-氟二苯甲酮的光取代反应中,C-卤素键如何断裂?
的有效photosubstitution反应米-3'-氟二苯酮及相关的photohydration反应在酸性水溶液中进行了系统的研究。飞秒瞬态吸收光谱法和纳秒时间分辨共振拉曼光谱法直接表征了其机理和中间体,而密度泛函理论计算则支持了这一过程。根据对元的观察,发现这种光解是一个两步过程-水合中间体。如对激发的三重态质子化物质的吸收光谱的观察所表明,确认了酮的质子化是进一步光化学反应的关键前体步骤。更有趣的是,有效的光解反应可以在特定条件下选择性地发生。进行了一系列卤素取代的二苯甲酮的对照实验,研究了溶液的酸度,取代基的位置以及取代的卤素的种类对形成相应的羟基光解产物的效率的影响。总结了预测目标光解反应效率的一些实际条件,并且成功地将它们用于预测其他一些卤素取代的二苯甲酮衍生物的光吸收反应何时发生。这种光解反应的驱动力可以提供对几种可能的应用的见解,这些应用也作了简要讨论。
更新日期:2015-09-14
中文翻译:

在酸性水溶液中3-氟二苯甲酮的光取代反应中,C-卤素键如何断裂?
的有效photosubstitution反应米-3'-氟二苯酮及相关的photohydration反应在酸性水溶液中进行了系统的研究。飞秒瞬态吸收光谱法和纳秒时间分辨共振拉曼光谱法直接表征了其机理和中间体,而密度泛函理论计算则支持了这一过程。根据对元的观察,发现这种光解是一个两步过程-水合中间体。如对激发的三重态质子化物质的吸收光谱的观察所表明,确认了酮的质子化是进一步光化学反应的关键前体步骤。更有趣的是,有效的光解反应可以在特定条件下选择性地发生。进行了一系列卤素取代的二苯甲酮的对照实验,研究了溶液的酸度,取代基的位置以及取代的卤素的种类对形成相应的羟基光解产物的效率的影响。总结了预测目标光解反应效率的一些实际条件,并且成功地将它们用于预测其他一些卤素取代的二苯甲酮衍生物的光吸收反应何时发生。这种光解反应的驱动力可以提供对几种可能的应用的见解,这些应用也作了简要讨论。































 京公网安备 11010802027423号
京公网安备 11010802027423号