当前位置:
X-MOL 学术
›
Chemosphere
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrophobic networked PbO2 electrode for electrochemical oxidation of paracetamol drug and degradation mechanism kinetics
Chemosphere ( IF 8.1 ) Pub Date : 2017-10-27 , DOI: 10.1016/j.chemosphere.2017.10.144 Yapeng He , Xue Wang , Weimin Huang , Rongling Chen , Wenli Zhang , Hongdong Li , Haibo Lin
Chemosphere ( IF 8.1 ) Pub Date : 2017-10-27 , DOI: 10.1016/j.chemosphere.2017.10.144 Yapeng He , Xue Wang , Weimin Huang , Rongling Chen , Wenli Zhang , Hongdong Li , Haibo Lin
|
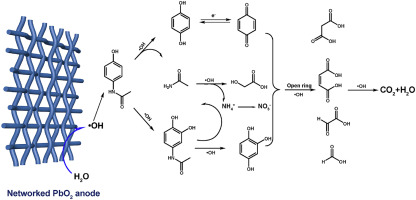
A hydrophobic networked PbO2 electrode was deposited on mesh titanium substrate and utilized for the electrochemical elimination towards paracetamol drug. Three dimensional growth mechanism of PbO2 layer provided more loading capacity of active materials and network structure greatly reduced the mass transfer for the electrochemical degradation. The active electrochemical surface area based on voltammetric charge quantity of networked PbO2 electrode is about 2.1 times for traditional PbO2 electrode while lower charge transfer resistance (6.78 Ω cm2) could be achieved on networked PbO2 electrode. The electrochemical incineration kinetics of paracetamol drug followed a pseudo first-order behavior and the corresponding rate constant were 0.354, 0.658 and 0.880 h−1 for traditional, networked PbO2 and boron doped diamond electrode. Higher electrochemical elimination kinetics could be achieved on networked PbO2 electrode and the performance can be equal to boron doped diamond electrode in result. Based on the quantification of reactive oxidants (hydroxyl radicals), the utilization rate of hydroxyl radicals could reach as high as 90% on networked PbO2 electrode. The enhancement of excellent electrochemical oxidation capacity towards paracetamol drug was related to the properties of higher loading capacity, enhanced mass transfer and hydrophobic surface. The possible degradation mechanism and pathway of paracetamol on networked PbO2 electrode were proposed in details accordingly based on the intermediate products.
中文翻译:

疏水性联网的PbO 2电极对乙酰氨基酚药物的电化学氧化及降解机理动力学
将疏水性网络状PbO 2电极沉积在网状钛基底上,并用于对乙酰氨基酚药物的电化学消除。PbO 2层的三维生长机理提供了更多的活性材料负载能力,网络结构大大降低了电化学降解的传质。基于网络化PbO 2电极的伏安电荷量的活性电化学表面积是传统PbO 2电极的约2.1倍,而网络化PbO 2可以实现更低的电荷转移电阻(6.78Ωcm 2)电极。对乙酰氨基酚药物的电化学焚烧动力学遵循伪一级行为,对于传统的网络化PbO 2和掺硼金刚石电极,其对应的速率常数分别为0.354、0.658和0.880 h -1。网络化的PbO 2电极可实现更高的电化学消除动力学,其性能可与掺硼金刚石电极媲美。通过对反应性氧化剂(羟基自由基)的定量分析,在网络化的PbO 2上羟基自由基的利用率可高达90%电极。对扑热息痛药物优异的电化学氧化能力的增强与更高的负载能力,增强的传质和疏水性表面有关。根据中间产物,详细提出了扑热息痛在网络化PbO 2电极上的可能降解机理和途径。
更新日期:2017-10-27
中文翻译:

疏水性联网的PbO 2电极对乙酰氨基酚药物的电化学氧化及降解机理动力学
将疏水性网络状PbO 2电极沉积在网状钛基底上,并用于对乙酰氨基酚药物的电化学消除。PbO 2层的三维生长机理提供了更多的活性材料负载能力,网络结构大大降低了电化学降解的传质。基于网络化PbO 2电极的伏安电荷量的活性电化学表面积是传统PbO 2电极的约2.1倍,而网络化PbO 2可以实现更低的电荷转移电阻(6.78Ωcm 2)电极。对乙酰氨基酚药物的电化学焚烧动力学遵循伪一级行为,对于传统的网络化PbO 2和掺硼金刚石电极,其对应的速率常数分别为0.354、0.658和0.880 h -1。网络化的PbO 2电极可实现更高的电化学消除动力学,其性能可与掺硼金刚石电极媲美。通过对反应性氧化剂(羟基自由基)的定量分析,在网络化的PbO 2上羟基自由基的利用率可高达90%电极。对扑热息痛药物优异的电化学氧化能力的增强与更高的负载能力,增强的传质和疏水性表面有关。根据中间产物,详细提出了扑热息痛在网络化PbO 2电极上的可能降解机理和途径。































 京公网安备 11010802027423号
京公网安备 11010802027423号