当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Computational Study Investigating the Energetics and Kinetics of the HNCO + (CH3)2NH Reaction Catalyzed by a Single Water Molecule
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-10-26 00:00:00 , DOI: 10.1021/acs.jpca.7b08657
Arathala Parandaman 1 , Chanin B. Tangtartharakul 1 , Manoj Kumar 2 , Joseph S. Francisco 2 , Amitabha Sinha 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-10-26 00:00:00 , DOI: 10.1021/acs.jpca.7b08657
Arathala Parandaman 1 , Chanin B. Tangtartharakul 1 , Manoj Kumar 2 , Joseph S. Francisco 2 , Amitabha Sinha 1
Affiliation
|
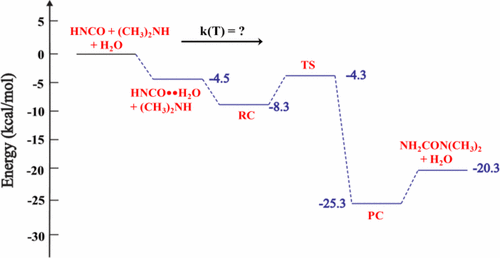
High-level ab initio calculations are used to explore the energetics and kinetics for the formation of 1,1-dimethyl urea via the reaction of isocyanic acid (HNCO) with dimethyl amine (DMA) catalyzed by a single water molecule. Compared to the uncatalyzed HNCO + DMA reaction, the presence of a water molecule lowers the reaction barrier, defined here as the energy difference between the separated HNCO + DMA + H2O reactants and the transition state (TS), by ∼26 kcal/mol. In addition to the HNCO + DMA + H2O reaction, the energetics of the analogous reactions involving, respectively, ammonia and methyl amine were also investigated. Comparing the barriers for these three amine addition reactions, which can be represented as HNCO + R-NH-R′ + H2O with R and R′ being either −CH3 or −H, we find that the reaction barrier decreases with the degree of methylation on the amine nitrogen atom. The effective rate constants for the bimolecular reaction pathways HNCO··H2O + DMA and HNCO··DMA + H2O were calculated using canonical variational TS theory coupled with both small curvature and zero-curvature tunneling corrections over the 200–300 K temperature range. For comparison, we also calculated the rate constant for the HNCO + OH reaction. Our results suggest that the HNCO + H2O + DMA reaction can make a non-negligible contribution to the gas-phase removal of atmospheric HNCO under conditions where the HNCO and water concentrations are high and the temperature is low.
中文翻译:

水分子催化HNCO +(CH 3)2 NH反应的能量和动力学的计算研究。
高水平的从头算是用来研究通过单个水分子催化的异氰酸(HNCO)与二甲胺(DMA)反应形成1,1-二甲基尿素的能量学和动力学。与未催化的HNCO + DMA反应相比,水分子的存在降低了反应势垒,在此定义为分离的HNCO + DMA + H 2 O反应物与过渡态(TS)之间的能量差约26 kcal /摩尔 除了HNCO + DMA + H 2 O反应之外,还研究了分别涉及氨和甲胺的类似反应的能级。比较这三个胺加成反应的障碍,可以将其表示为HNCO + R-NH-R'+ H 2当R和R'为-CH 3或-H的O时,我们发现反应障碍随着胺氮原子上甲基化程度的降低而降低。使用规范变分TS理论,结合小曲率和零曲率隧道校正,在200–300 K范围内,计算了双分子反应路径HNCO··H 2 O + DMA和HNCO··DMA + H 2 O的有效速率常数温度范围。为了进行比较,我们还计算了HNCO + OH反应的速率常数。我们的结果表明,在HNCO和水浓度高而温度低的条件下,HNCO + H 2 O + DMA反应对大气HNCO气相去除的贡献不可忽略。
更新日期:2017-10-27
中文翻译:

水分子催化HNCO +(CH 3)2 NH反应的能量和动力学的计算研究。
高水平的从头算是用来研究通过单个水分子催化的异氰酸(HNCO)与二甲胺(DMA)反应形成1,1-二甲基尿素的能量学和动力学。与未催化的HNCO + DMA反应相比,水分子的存在降低了反应势垒,在此定义为分离的HNCO + DMA + H 2 O反应物与过渡态(TS)之间的能量差约26 kcal /摩尔 除了HNCO + DMA + H 2 O反应之外,还研究了分别涉及氨和甲胺的类似反应的能级。比较这三个胺加成反应的障碍,可以将其表示为HNCO + R-NH-R'+ H 2当R和R'为-CH 3或-H的O时,我们发现反应障碍随着胺氮原子上甲基化程度的降低而降低。使用规范变分TS理论,结合小曲率和零曲率隧道校正,在200–300 K范围内,计算了双分子反应路径HNCO··H 2 O + DMA和HNCO··DMA + H 2 O的有效速率常数温度范围。为了进行比较,我们还计算了HNCO + OH反应的速率常数。我们的结果表明,在HNCO和水浓度高而温度低的条件下,HNCO + H 2 O + DMA反应对大气HNCO气相去除的贡献不可忽略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号