当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New Insights into the Performance Degradation of Fe-Based Layered Oxides in Sodium-Ion Batteries: Instability of Fe3+/Fe4+ Redox in α-NaFeO2
Chemistry of Materials ( IF 7.2 ) Pub Date : 2015-09-11 00:00:00 , DOI: 10.1021/acs.chemmater.5b02918
Eungje Lee , Dennis E. Brown 1 , Esen E. Alp , Yang Ren , Jun Lu , Jung-Je Woo , Christopher S. Johnson
Chemistry of Materials ( IF 7.2 ) Pub Date : 2015-09-11 00:00:00 , DOI: 10.1021/acs.chemmater.5b02918
Eungje Lee , Dennis E. Brown 1 , Esen E. Alp , Yang Ren , Jun Lu , Jung-Je Woo , Christopher S. Johnson
Affiliation
|
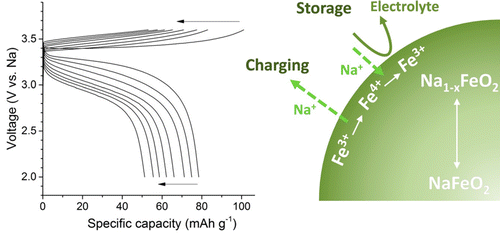
The emergence of sodium-ion batteries (SIBs) employing cathodes based on earth abundant sodium and iron is expected to be ideal for large-scale electrical energy storage systems, for which the cost factor is of primary importance. However, these iron-based layered oxides still show unsatisfactory cycle performance, and the redox of the fleeting Fe3+/Fe4+ couple needs to be better understood. In this study, we examine the quasi-reversibility of the layered α-NaFeO2 cathode in sodium-ion cells. A NaFeO2 powder sample that has the O3-type layered structure was synthesized via a solid-state synthesis method. The changes in Fe oxidation states and crystallographic structures were examined during the electrochemical sodium cycling of the NaFeO2 electrodes. Ex situ Mössbauer spectroscopy analysis revealed the chemical instability of Fe4+ in a battery cell environment: more than 20% of Fe4+ species that was generated in the desodiated Na1–xFeO2 electrode was spontaneously reduced back to Fe3+ states during open circuit storage of the charged cell. The in situ synchrotron X-ray diffraction further revealed the nonequilibrium phase transition behavior of the NaFeO2 cathode. A new layered phase (denoted as O″3) was observed in the course of sodium deintercalation, and an asymmetric structural behavior during cycling was identified. These findings explain the quasi-reversibility of α-NaFeO2 in the sodium cell and provide guidance for the future development of iron-based cathode materials for sodium-ion batteries.
中文翻译:

新的洞察铁基层状氧化物的钠离子电池性能下降:铁的不稳定3+ / Fe的4+在氧化还原α-NaFeO 2
预计采用基于富含钠和铁的阴极的钠离子电池(SIB)的出现对于大型电能存储系统是理想的选择,对于这些系统而言,成本因素至关重要。然而,这些铁基层状氧化物仍显示出不令人满意的循环性能,并且需要更好地理解短暂的Fe 3+ / Fe 4+对的氧化还原。在这项研究中,我们检查层状α-NaFeO的准可逆性2中钠离子电池阴极。具有O3的NaFeO 2粉末样品通过固态合成方法合成了α-型层状结构。在NaFeO 2电极的电化学钠循环过程中检查了Fe氧化态和晶体结构的变化。异位Mössbauer光谱分析揭示了电池单元环境中Fe 4+的化学不稳定性:在经过去离子处理的Na 1– x FeO 2电极中生成的超过20%的Fe 4+物种自发还原为Fe 3+态在带电电池的开路存储期间。原位同步加速器X射线衍射进一步揭示了NaFeO 2的非平衡相变行为阴极。一种新的层状相(表示为○“3中脱嵌钠的过程中,观察到),并且在循环过程中的非对称结构的行为被确定。这些发现解释α-NaFeO的准可逆性2在钠电池和用于铁基的阴极材料为钠离子电池的未来发展提供指导。
更新日期:2015-09-11
中文翻译:

新的洞察铁基层状氧化物的钠离子电池性能下降:铁的不稳定3+ / Fe的4+在氧化还原α-NaFeO 2
预计采用基于富含钠和铁的阴极的钠离子电池(SIB)的出现对于大型电能存储系统是理想的选择,对于这些系统而言,成本因素至关重要。然而,这些铁基层状氧化物仍显示出不令人满意的循环性能,并且需要更好地理解短暂的Fe 3+ / Fe 4+对的氧化还原。在这项研究中,我们检查层状α-NaFeO的准可逆性2中钠离子电池阴极。具有O3的NaFeO 2粉末样品通过固态合成方法合成了α-型层状结构。在NaFeO 2电极的电化学钠循环过程中检查了Fe氧化态和晶体结构的变化。异位Mössbauer光谱分析揭示了电池单元环境中Fe 4+的化学不稳定性:在经过去离子处理的Na 1– x FeO 2电极中生成的超过20%的Fe 4+物种自发还原为Fe 3+态在带电电池的开路存储期间。原位同步加速器X射线衍射进一步揭示了NaFeO 2的非平衡相变行为阴极。一种新的层状相(表示为○“3中脱嵌钠的过程中,观察到),并且在循环过程中的非对称结构的行为被确定。这些发现解释α-NaFeO的准可逆性2在钠电池和用于铁基的阴极材料为钠离子电池的未来发展提供指导。

































 京公网安备 11010802027423号
京公网安备 11010802027423号