当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Dearomative Halogenation of β-Naphthols: The Axial Chirality Transfer Reaction
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-10-24 06:34:34 , DOI: 10.1002/adsc.201700745 Pengxin Wang 1 , Jie Wang 1 , Linqing Wang 1 , Dan Li 1 , Kezhou Wang 1 , Yuyang Liu 1 , Haiyong Zhu 1 , Xihong Liu 1 , Dongxu Yang 1 , Rui Wang 1, 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-10-24 06:34:34 , DOI: 10.1002/adsc.201700745 Pengxin Wang 1 , Jie Wang 1 , Linqing Wang 1 , Dan Li 1 , Kezhou Wang 1 , Yuyang Liu 1 , Haiyong Zhu 1 , Xihong Liu 1 , Dongxu Yang 1 , Rui Wang 1, 2
Affiliation
|
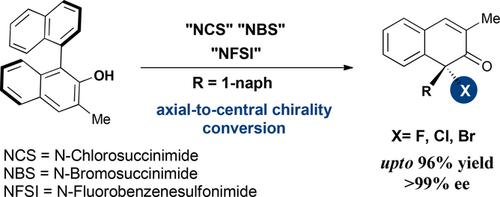
Axial naphthols are applied in asymmetric halogenative dearomatization reactions under simple and mild conditions in the work presented herein. The axial-to-central chirality conversion is efficiently accomplished, and the desired halogenated dearomatization products are obtained in high yields and enantioselectivities. By using commercially available halogenation reagents, the asymmetric fluorinative, chlorinative and brominative dearomatization reactions of axial naphthols derived from BINOLs are achieved. (BINOL=1,1′-Bi-2-naphthol)
中文翻译:

β-萘酚的不对称脱芳香族卤代反应:轴向手性转移反应
在本文介绍的工作中,轴向萘酚在简单和温和的条件下用于不对称的卤化脱芳香化反应中。有效地完成了从轴向到中心的手性转化,并以高收率和对映选择性获得了所需的卤代脱芳香化产物。通过使用市售的卤化试剂,可以实现衍生自BINOL的轴向萘的不对称氟化,氯化和溴化脱芳香化反应。(BINOL = 1,1'-Bi-2-萘酚)
更新日期:2017-10-24
中文翻译:

β-萘酚的不对称脱芳香族卤代反应:轴向手性转移反应
在本文介绍的工作中,轴向萘酚在简单和温和的条件下用于不对称的卤化脱芳香化反应中。有效地完成了从轴向到中心的手性转化,并以高收率和对映选择性获得了所需的卤代脱芳香化产物。通过使用市售的卤化试剂,可以实现衍生自BINOL的轴向萘的不对称氟化,氯化和溴化脱芳香化反应。(BINOL = 1,1'-Bi-2-萘酚)































 京公网安备 11010802027423号
京公网安备 11010802027423号