当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Control of α/β Anomer Formation by a 2′,5′ Bridge: Toward Nucleoside Derivatives Locked in the South Conformation
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-10-24 00:00:00 , DOI: 10.1021/acs.joc.7b01000 Hubert Hřebabecký 1 , Martin Dračínský 1 , Eliška Procházková 1 , Michal Šála 1 , Richard Mackman 2 , Radim Nencka 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-10-24 00:00:00 , DOI: 10.1021/acs.joc.7b01000 Hubert Hřebabecký 1 , Martin Dračínský 1 , Eliška Procházková 1 , Michal Šála 1 , Richard Mackman 2 , Radim Nencka 1
Affiliation
|
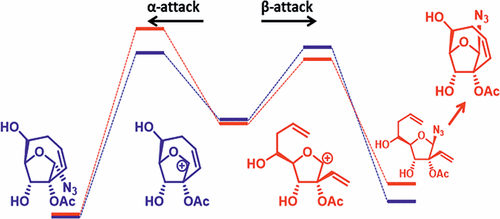
We describe a novel stereoselective synthesis of nucleoside derivatives with the ribose ring locked in the South conformation by a bridge between C2′ and C5′. Despite the intrinsic constraints of the bicyclic structure, we demonstrate that their synthesis can be achieved by ring closing metathesis of readily accessible precursors. The obtained ribose derivatives are, however, very poor substrates for further installation of the nucleobases, and even simple nucleophiles, such as azido or cyano anions, react with unexpected stereo- or regioselectivity under standard glycosylation conditions. Here we explain this behavior by employing density functional theory (DFT) computations and devise an alternative approach resulting in isomers with the desired orientation of the nucleobase.
中文翻译:

通过2',5'桥控制α/β端基异构体的形成:锁定在南构象中的核苷衍生物
我们描述了核苷衍生物的新型立体选择性合成,其核糖环通过C2'和C5'之间的桥锁定在South构象中。尽管存在双环结构的固有限制,但我们证明了它们的合成可以通过容易获得的前体的闭环复分解来实现。然而,获得的核糖衍生物是用于进一步安装核碱基的非常差的底物,甚至简单的亲核试剂,例如叠氮基或氰基阴离子,在标准糖基化条件下也会与意想不到的立体选择性或区域选择性反应。在这里,我们通过采用密度泛函理论(DFT)计算来解释这种行为,并设计出一种替代方法,使异构体具有所需的核碱基方向。
更新日期:2017-10-25
中文翻译:

通过2',5'桥控制α/β端基异构体的形成:锁定在南构象中的核苷衍生物
我们描述了核苷衍生物的新型立体选择性合成,其核糖环通过C2'和C5'之间的桥锁定在South构象中。尽管存在双环结构的固有限制,但我们证明了它们的合成可以通过容易获得的前体的闭环复分解来实现。然而,获得的核糖衍生物是用于进一步安装核碱基的非常差的底物,甚至简单的亲核试剂,例如叠氮基或氰基阴离子,在标准糖基化条件下也会与意想不到的立体选择性或区域选择性反应。在这里,我们通过采用密度泛函理论(DFT)计算来解释这种行为,并设计出一种替代方法,使异构体具有所需的核碱基方向。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号