当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Crystallization-Induced Emission Enhancement of a Simple Tolane-Based Mesogenic Luminogen
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2015-09-10 00:00:00 , DOI: 10.1021/acs.jpcc.5b06088
Jiaqi Tong 1 , Yi Jia Wang 1 , Zhaoyang Wang 1 , Jing Zhi Sun 1 , Ben Zhong Tang 1, 2, 3
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2015-09-10 00:00:00 , DOI: 10.1021/acs.jpcc.5b06088
Jiaqi Tong 1 , Yi Jia Wang 1 , Zhaoyang Wang 1 , Jing Zhi Sun 1 , Ben Zhong Tang 1, 2, 3
Affiliation
|
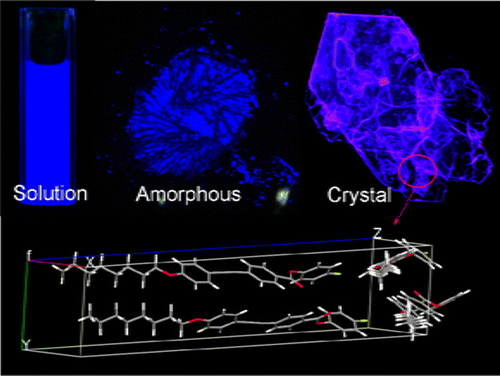
The photophysical properties of a diphenylacetylene derivative (FOEB) were investigated. The fluorescence quantum efficiency (ΦF, %) of FOEB changes from moderate (49%) to low (9%) and to evidently enhanced (60%) in dilute solution, amorphous solid, and crystal state, respectively. This is a typical phenomenon of crystallization-induced emission enhancement (CIEE). The diphenylacetylene luminogen can be viewed as a propeller-like molecule with two blades (phenyl groups). In solution, the intramolecular rotations of the two blades around the ethynyl unit are activated and partially dissipate the energy in the photoexcited state, thereby leading to emission quenching. Consequently, FOEB exhibits moderate ΦF in solution. In amorphous solid, the intramolecular rotations are restricted, and a higher ΦF could be expected. But in fact, the strong π–π interactions between the luminogens cause heavier emission quenching, and the ΦF of the molecular aggregates is as low as 9%. In the single crystal, as the crystallographic data revealed, the two phenyl groups in the same diphenylacetylene unit are highly coplanar and the conjugation planes of the two adjacent diphenylacetylene units are orthogonal to each other. Such molecular conformation and chromophore packing eliminate the intermolecular π–π interaction thereby greatly reducing the π–π interaction that caused fluorescence quenching, and meanwhile, the orthogonal packing allows the formation of C–H···π bonding between the adjacent phenyl groups to lock the intramolecular rotations and retain fluorescence. As a result, the FOEB single crystal shows evidently enhanced emission. Besides the CIEE property, FOEB demonstrates good liquid crystal property due to the diphenylacetylene mesogen and solvatochromic and mechnochromic effects due to the modification of diphenylacetylene with introduction of electron donor and acceptor functionalities.
中文翻译:

结晶诱导的简单的基于Tolane的介晶发光剂的发射增强
研究了二苯乙炔衍生物(FOEB)的光物理性质。荧光量子效率(Φ ˚F,%)FOEB的中度(49%)到低(9%)和对明显提高(60%)在稀溶液中,无定形固体,和结晶状态,分别改变。这是结晶诱导的发射增强(CIEE)的典型现象。联苯乙炔发光剂可以看作是带有两个叶片(苯基)的螺旋桨状分子。在溶液中,两个叶片绕乙炔基单元的分子内旋转被激活,并在光激发态下部分耗散能量,从而导致发射猝灭。因此,FOEB展品中度Φ ˚F在解决方案中。在无定形固体中,分子内的旋转受到限制,和较高的Φ ˚F可以预期。但在实际上,luminogens之间的强π-π相互作用导致较重的发射猝灭,并且Φ ˚F分子聚集体的10%低至9%。在单晶中,如晶体学数据所示,同一二苯乙炔单元中的两个苯基高度共面,并且两个相邻的二苯乙炔单元的共轭面彼此正交。这种分子构象和发色团堆积消除了分子间的π-π相互作用,从而大大减少了引起荧光猝灭的π-π相互作用,同时,正交堆积使相邻苯基之间形成C–H···π键,锁定分子内旋转并保留荧光。结果,FOEB单晶显示出明显增强的发射。除了CIEE属性,
更新日期:2015-09-10
中文翻译:

结晶诱导的简单的基于Tolane的介晶发光剂的发射增强
研究了二苯乙炔衍生物(FOEB)的光物理性质。荧光量子效率(Φ ˚F,%)FOEB的中度(49%)到低(9%)和对明显提高(60%)在稀溶液中,无定形固体,和结晶状态,分别改变。这是结晶诱导的发射增强(CIEE)的典型现象。联苯乙炔发光剂可以看作是带有两个叶片(苯基)的螺旋桨状分子。在溶液中,两个叶片绕乙炔基单元的分子内旋转被激活,并在光激发态下部分耗散能量,从而导致发射猝灭。因此,FOEB展品中度Φ ˚F在解决方案中。在无定形固体中,分子内的旋转受到限制,和较高的Φ ˚F可以预期。但在实际上,luminogens之间的强π-π相互作用导致较重的发射猝灭,并且Φ ˚F分子聚集体的10%低至9%。在单晶中,如晶体学数据所示,同一二苯乙炔单元中的两个苯基高度共面,并且两个相邻的二苯乙炔单元的共轭面彼此正交。这种分子构象和发色团堆积消除了分子间的π-π相互作用,从而大大减少了引起荧光猝灭的π-π相互作用,同时,正交堆积使相邻苯基之间形成C–H···π键,锁定分子内旋转并保留荧光。结果,FOEB单晶显示出明显增强的发射。除了CIEE属性,

































 京公网安备 11010802027423号
京公网安备 11010802027423号