当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrocatalytic Radical Dichlorination of Alkenes with Nucleophilic Chlorine Sources
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-10-23 00:00:00 , DOI: 10.1021/jacs.7b09388 Niankai Fu 1 , Gregory S. Sauer 1 , Song Lin 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-10-23 00:00:00 , DOI: 10.1021/jacs.7b09388 Niankai Fu 1 , Gregory S. Sauer 1 , Song Lin 1, 2
Affiliation
|
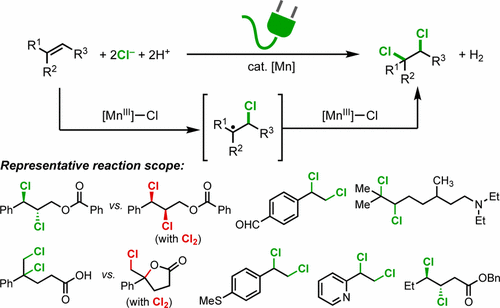
We report a Mn-catalyzed electrochemical dichlorination of alkenes with MgCl2 as the chlorine source. This method provides operationally simple, sustainable, and efficient access to a variety of vicinally dichlorinated compounds. In particular, alkenes with oxidatively labile functional groups, such as alcohols, aldehydes, sulfides, and amines, were transformed into the desired vicinal dichlorides with high chemoselectivity. Mechanistic data are consistent with metal-mediated Cl atom transfer as the predominant pathway enabling dual C–Cl bond formation and contradict an alternative pathway involving electrochemical evolution of chlorine gas followed by Cl2-mediated electrophilic dichlorination.
中文翻译:

亲核氯源对烯烃的电催化自由基二氯化
我们报告了锰催化的MgCl 2作为氯源的烯烃的电化学二氯化。该方法提供了操作上简单,可持续和有效的途径,可接触多种二氯氯代化合物。特别是,将具有氧化不稳定官能团的烯烃,例如醇,醛,硫化物和胺,以高化学选择性转化为所需的邻位二氯化物。机理数据与金属介导的Cl原子转移相一致,因为其主要途径可实现双C–Cl键形成,并且与涉及氯气的电化学逸出然后由Cl 2介导的亲电二氯化作用的另一种途径相矛盾。
更新日期:2017-10-24
中文翻译:

亲核氯源对烯烃的电催化自由基二氯化
我们报告了锰催化的MgCl 2作为氯源的烯烃的电化学二氯化。该方法提供了操作上简单,可持续和有效的途径,可接触多种二氯氯代化合物。特别是,将具有氧化不稳定官能团的烯烃,例如醇,醛,硫化物和胺,以高化学选择性转化为所需的邻位二氯化物。机理数据与金属介导的Cl原子转移相一致,因为其主要途径可实现双C–Cl键形成,并且与涉及氯气的电化学逸出然后由Cl 2介导的亲电二氯化作用的另一种途径相矛盾。































 京公网安备 11010802027423号
京公网安备 11010802027423号