Molecular Cell ( IF 14.5 ) Pub Date : 2017-10-19 , DOI: 10.1016/j.molcel.2017.09.026
Kuang Shen 1 , Abigail Choe 2 , David M Sabatini 1
|
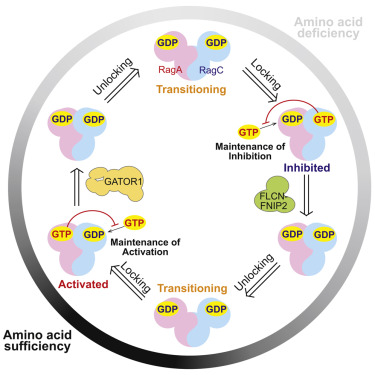
mTOR complex I (mTORC1) is a central growth regulator that senses amino acids through a pathway that converges on the Rag GTPases, an obligate heterodimer of two related GTPases. Despite their central role in amino acid sensing, it is unknown why the Rag GTPases are heterodimeric and whether their subunits communicate with each other. Here, we find that the binding of guanosine triphosphate (GTP) to one subunit inhibits the binding and induces the hydrolysis of GTP by the other. This intersubunit communication pushes the Rag GTPases into either of two stable configurations, which represent active “on” or “off” states that interconvert via transient intermediates. Subunit coupling confers on the mTORC1 pathway its capacity to respond rapidly to the amino acid level. Thus, the dynamic response of mTORC1 requires intersubunit communication by the Rag GTPases, providing a rationale for why they exist as a dimer and revealing a distinct mode of control for a GTP-binding protein.
中文翻译:

Rag GTPase 异二聚体中的亚基间串扰使 mTORC1 能够快速响应氨基酸可用性
mTOR 复合物 I (mTORC1) 是一种中央生长调节剂,通过汇聚 Rag GTPases(两种相关 GTPases 的专性异二聚体)的途径感知氨基酸。尽管 Rag GTP 酶在氨基酸传感中发挥核心作用,但仍不清楚为什么 Rag GTP 酶是异二聚体以及它们的亚基是否相互通信。在这里,我们发现三磷酸鸟苷 (GTP) 与一个亚基的结合会抑制该结合并诱导另一个亚基水解 GTP。这种子单元间通信将 Rag GTPases 推入两种稳定配置中的任一种,这两种稳定配置代表通过瞬态中间体相互转换的活跃“开”或“关”状态。亚基偶联赋予 mTORC1 通路快速响应氨基酸水平的能力。因此,mTORC1 的动态响应需要 Rag GTPases 进行亚基间通讯,这为它们以二聚体形式存在提供了基本原理,并揭示了 GTP 结合蛋白的独特控制模式。

































 京公网安备 11010802027423号
京公网安备 11010802027423号