当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Macrophage VLDLR mediates obesity-induced insulin resistance with adipose tissue inflammation.
Nature Communications ( IF 14.7 ) Pub Date : 2017-10-20 , DOI: 10.1038/s41467-017-01232-w
Kyung Cheul Shin , Injae Hwang , Sung Sik Choe , Jeu Park , Yul Ji , Jong In Kim , Gha Young Lee , Sung Hee Choi , Jianhong Ching , Jean-Paul Kovalik , Jae Bum Kim
Nature Communications ( IF 14.7 ) Pub Date : 2017-10-20 , DOI: 10.1038/s41467-017-01232-w
Kyung Cheul Shin , Injae Hwang , Sung Sik Choe , Jeu Park , Yul Ji , Jong In Kim , Gha Young Lee , Sung Hee Choi , Jianhong Ching , Jean-Paul Kovalik , Jae Bum Kim
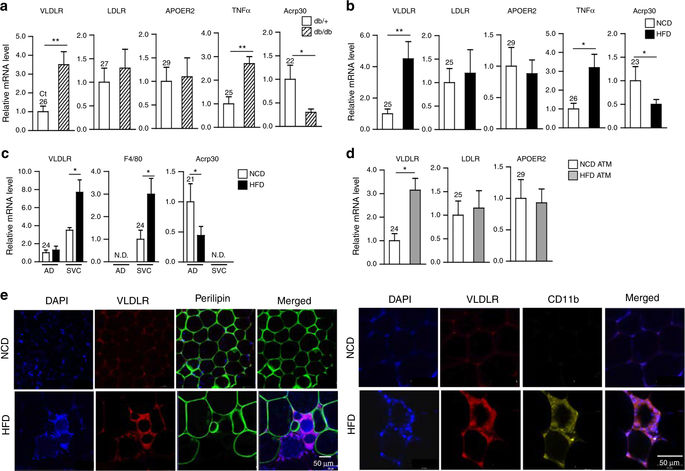
|
Obesity is closely associated with increased adipose tissue macrophages (ATMs), which contribute to systemic insulin resistance and altered lipid metabolism by creating a pro-inflammatory environment. Very low-density lipoprotein receptor (VLDLR) is involved in lipoprotein uptake and storage. However, whether lipid uptake via VLDLR in macrophages affects obesity-induced inflammatory responses and insulin resistance is not well understood. Here we show that elevated VLDLR expression in ATMs promotes adipose tissue inflammation and glucose intolerance in obese mice. In macrophages, VLDL treatment upregulates intracellular levels of C16:0 ceramides in a VLDLR-dependent manner, which potentiates pro-inflammatory responses and promotes M1-like macrophage polarization. Adoptive transfer of VLDLR knockout bone marrow to wild-type mice relieves adipose tissue inflammation and improves insulin resistance in diet-induced obese mice. These findings suggest that increased VLDL-VLDLR signaling in ATMs aggravates adipose tissue inflammation and insulin resistance in obesity.
中文翻译:

巨噬细胞VLDLR介导肥胖诱导的胰岛素抵抗与脂肪组织炎症。
肥胖与增加的脂肪组织巨噬细胞(ATM)密切相关,后者通过产生促炎性环境而导致全身性胰岛素抵抗和脂质代谢改变。非常低密度的脂蛋白受体(VLDLR)参与脂蛋白的摄取和储存。然而,尚不完全了解通过巨噬细胞中的VLDLR摄取脂质是否会影响肥胖引起的炎症反应和胰岛素抵抗。在这里,我们显示,ATM中VLDLR的表达升高可促进肥胖小鼠的脂肪组织炎症和葡萄糖耐量下降。在巨噬细胞中,VLDL治疗以VLDLR依赖性方式上调C16:0神经酰胺的细胞内水平,从而增强促炎反应并促进M1样巨噬细胞极化。VLDLR基因敲除骨髓向野生型小鼠的过继转移减轻了脂肪组织的炎症,并改善了饮食诱导的肥胖小鼠的胰岛素抵抗。这些发现表明,ATM中VLDL-VLDLR信号的增加会加剧肥胖患者的脂肪组织炎症和胰岛素抵抗。
更新日期:2017-10-20
中文翻译:

巨噬细胞VLDLR介导肥胖诱导的胰岛素抵抗与脂肪组织炎症。
肥胖与增加的脂肪组织巨噬细胞(ATM)密切相关,后者通过产生促炎性环境而导致全身性胰岛素抵抗和脂质代谢改变。非常低密度的脂蛋白受体(VLDLR)参与脂蛋白的摄取和储存。然而,尚不完全了解通过巨噬细胞中的VLDLR摄取脂质是否会影响肥胖引起的炎症反应和胰岛素抵抗。在这里,我们显示,ATM中VLDLR的表达升高可促进肥胖小鼠的脂肪组织炎症和葡萄糖耐量下降。在巨噬细胞中,VLDL治疗以VLDLR依赖性方式上调C16:0神经酰胺的细胞内水平,从而增强促炎反应并促进M1样巨噬细胞极化。VLDLR基因敲除骨髓向野生型小鼠的过继转移减轻了脂肪组织的炎症,并改善了饮食诱导的肥胖小鼠的胰岛素抵抗。这些发现表明,ATM中VLDL-VLDLR信号的增加会加剧肥胖患者的脂肪组织炎症和胰岛素抵抗。

































 京公网安备 11010802027423号
京公网安备 11010802027423号