Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Time-controllable Nkcc1 knockdown replicates reversible hearing loss in postnatal mice.
Scientific Reports ( IF 3.8 ) Pub Date : 2017-Oct-19 , DOI: 10.1038/s41598-017-13997-7
Takahisa Watabe 1 , Ming Xu 2 , Miho Watanabe 3 , Junichi Nabekura 4 , Taiga Higuchi 5 , Karin Hori 5 , Mitsuo P Sato 5 , Fumiaki Nin 5 , Hiroshi Hibino 5, 6 , Kaoru Ogawa 1 , Masatsugu Masuda 1, 7 , Kenji F Tanaka 2
Scientific Reports ( IF 3.8 ) Pub Date : 2017-Oct-19 , DOI: 10.1038/s41598-017-13997-7
Takahisa Watabe 1 , Ming Xu 2 , Miho Watanabe 3 , Junichi Nabekura 4 , Taiga Higuchi 5 , Karin Hori 5 , Mitsuo P Sato 5 , Fumiaki Nin 5 , Hiroshi Hibino 5, 6 , Kaoru Ogawa 1 , Masatsugu Masuda 1, 7 , Kenji F Tanaka 2
Affiliation
|
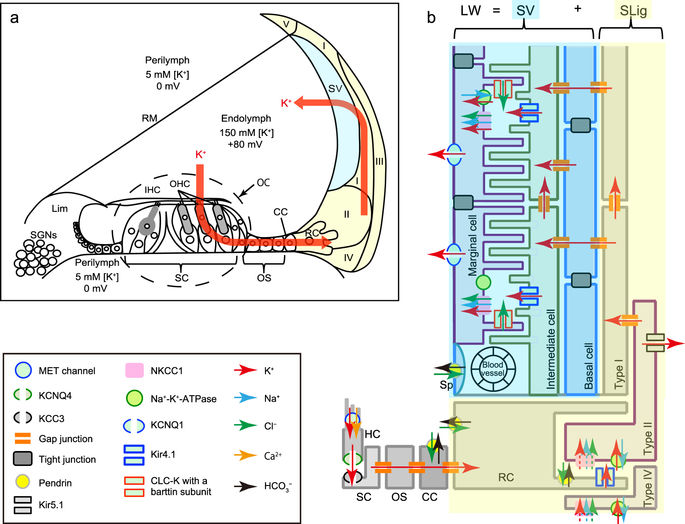
Identification of the causal effects of specific proteins on recurrent and partially reversible hearing loss has been difficult because of the lack of an animal model that provides reversible gene knockdown. We have developed the transgenic mouse line Actin-tTS::Nkcc1 tetO/tetO for manipulatable expression of the cochlear K+ circulation protein, NKCC1. Nkcc1 transcription was blocked by the binding of a tetracycline-dependent transcriptional silencer to the tetracycline operator sequences inserted upstream of the Nkcc1 translation initiation site. Administration of the tetracycline derivative doxycycline reversibly regulated Nkcc1 knockdown. Progeny from pregnant/lactating mothers fed doxycycline-free chow from embryonic day 0 showed strong suppression of Nkcc1 expression (~90% downregulation) and Nkcc1 null phenotypes at postnatal day 35 (P35). P35 transgenic mice from mothers fed doxycycline-free chow starting at P0 (delivery) showed weaker suppression of Nkcc1 expression (~70% downregulation) and less hearing loss with mild cochlear structural changes. Treatment of these mice at P35 with doxycycline for 2 weeks reactivated Nkcc1 transcription to control levels and improved hearing level at high frequency; i.e., these doxycycline-treated mice exhibited partially reversible hearing loss. Thus, development of the Actin-tTS::Nkcc1 tetO/tetO transgenic mouse line provides a mouse model for the study of variable hearing loss through reversible knockdown of Nkcc1.
中文翻译:

时间可控的 Nkcc1 敲低在产后小鼠中复制了可逆性听力损失。
由于缺乏提供可逆基因敲除的动物模型,识别特定蛋白质对复发性和部分可逆性听力损失的因果影响一直很困难。我们开发了转基因小鼠品系 Actin-tTS::Nkcc1 tetO/tetO,用于可操作地表达耳蜗 K +循环蛋白 NKCC1。 Nkcc1 转录通过四环素依赖性转录沉默子与插入 Nkcc1 翻译起始位点上游的四环素操纵子序列的结合而被阻断。四环素衍生物多西环素的施用可逆地调节 Nkcc1 敲低。从胚胎第 0 天开始喂养不含强力霉素的食物的怀孕/哺乳母亲的后代在出生后第 35 天表现出 Nkcc1 表达的强烈抑制(~90% 下调)和 Nkcc1 无效表型(P35)。从 P0(分娩)开始喂养不含强力霉素的母亲的 P35 转基因小鼠表现出较弱的 Nkcc1 表达抑制(约 70% 下调)和较少的听力损失,并伴有轻微的耳蜗结构变化。在 P35 时用多西环素治疗这些小鼠 2 周,重新激活 Nkcc1 转录以控制水平并改善高频听力水平;也就是说,这些用多西环素治疗的小鼠表现出部分可逆的听力损失。因此,Actin-tTS::Nkcc1 tetO/tetO转基因小鼠系的开发为通过可逆性敲低 Nkcc1 来研究可变性听力损失提供了小鼠模型。
更新日期:2017-10-19
中文翻译:

时间可控的 Nkcc1 敲低在产后小鼠中复制了可逆性听力损失。
由于缺乏提供可逆基因敲除的动物模型,识别特定蛋白质对复发性和部分可逆性听力损失的因果影响一直很困难。我们开发了转基因小鼠品系 Actin-tTS::Nkcc1 tetO/tetO,用于可操作地表达耳蜗 K +循环蛋白 NKCC1。 Nkcc1 转录通过四环素依赖性转录沉默子与插入 Nkcc1 翻译起始位点上游的四环素操纵子序列的结合而被阻断。四环素衍生物多西环素的施用可逆地调节 Nkcc1 敲低。从胚胎第 0 天开始喂养不含强力霉素的食物的怀孕/哺乳母亲的后代在出生后第 35 天表现出 Nkcc1 表达的强烈抑制(~90% 下调)和 Nkcc1 无效表型(P35)。从 P0(分娩)开始喂养不含强力霉素的母亲的 P35 转基因小鼠表现出较弱的 Nkcc1 表达抑制(约 70% 下调)和较少的听力损失,并伴有轻微的耳蜗结构变化。在 P35 时用多西环素治疗这些小鼠 2 周,重新激活 Nkcc1 转录以控制水平并改善高频听力水平;也就是说,这些用多西环素治疗的小鼠表现出部分可逆的听力损失。因此,Actin-tTS::Nkcc1 tetO/tetO转基因小鼠系的开发为通过可逆性敲低 Nkcc1 来研究可变性听力损失提供了小鼠模型。

































 京公网安备 11010802027423号
京公网安备 11010802027423号