Chem ( IF 19.1 ) Pub Date : 2017-10-19 , DOI: 10.1016/j.chempr.2017.09.012 João Ramiro Robalo , Susanne Huhmann , Beate Koksch , Ana Vila Verde
|
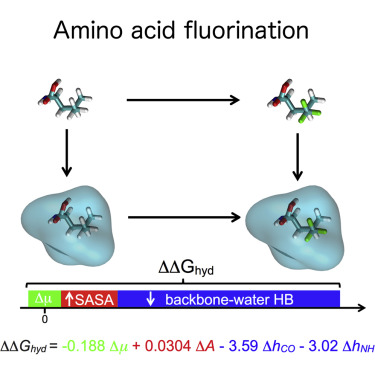
The substitution of –CH3 with –CF3 groups in the side chain of hydrophobic amino acids often increases their hydrophobicity, but the impact of these substitutions on the thermal stability of proteins is system specific. Here, we investigated this issue by using fixed-charge, all-atom molecular dynamics simulations and an AMBER-compatible library of fluorinated amino acids. We found that the changes in hydration free energy upon fluorination depended strongly on amino acid identity and the location of the fluorinated site. We present a phenomenological model that quantitatively predicts the simulation results. The model demonstrates that changes in hydrophobicity upon fluorination largely arise from steric hindrance of backbone-water hydrogen bonds by –CF3; changes in surface area, often invoked to explain experimental trends, have only a secondary contribution. The model and the force field presented here provide indispensable molecular-scale insight and simulation tools for understanding and predicting the impact of fluorination on protein properties.
中文翻译:

氟化非极性氨基酸疏水性的多种来源
在疏水性氨基酸的侧链中,用–CF 3基团取代–CH 3通常会增加其疏水性,但是这些取代对蛋白质热稳定性的影响是系统特异性的。在这里,我们通过使用固定电荷的全原子分子动力学模拟和AMBER兼容的氟化氨基酸库来研究此问题。我们发现氟化后水合自由能的变化在很大程度上取决于氨基酸身份和氟化位点的位置。我们提出了一种现象学模型,可以定量地预测模拟结果。该模型表明,氟化过程中疏水性的变化很大程度上是由–CF 3对主链-水氢键的空间位阻造成的。; 经常被用来解释实验趋势的表面积变化仅是次要的贡献。这里介绍的模型和力场提供了不可或缺的分子尺度见解和模拟工具,用于理解和预测氟化对蛋白质特性的影响。

































 京公网安备 11010802027423号
京公网安备 11010802027423号