当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis of Resiniferatoxin Enabled by Radical-Mediated Three-Component Coupling and 7-endo Cyclization
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-10-31 , DOI: 10.1021/jacs.7b10177 Satoshi Hashimoto 1 , Shun-ichiro Katoh 1 , Takehiro Kato 1 , Daisuke Urabe 1 , Masayuki Inoue 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-10-31 , DOI: 10.1021/jacs.7b10177 Satoshi Hashimoto 1 , Shun-ichiro Katoh 1 , Takehiro Kato 1 , Daisuke Urabe 1 , Masayuki Inoue 1
Affiliation
|
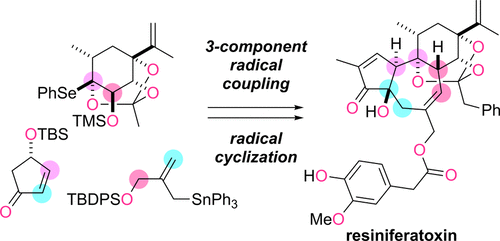
Resiniferatoxin (1) belongs to a daphnane diterpenoid family and has strong agonistic effects on TRPV1, a transducer of noxious temperature and chemical stimuli. The densely oxygenated trans-fused 5/7/6-tricarbocycle (ABC-ring) of 1 presents a daunting challenge for chemical synthesis. Here we report the development of a novel radical-based strategy for assembling 1 from three components: A-ring 9, allyl stannane 18b, and C-ring 17b. The 6-membered 17b, prepared from d-ribose derivative 19, was designed to possess the caged orthoester structure with α-alkoxy selenide as a radical precursor. Upon treatment of 17b with 18b, 9, and V-40, the potently reactive α-alkoxy bridgehead radical was generated from 17b and then sequentially coupled with 9 and 18b to yield 16b. This first radical reaction formed the hindered C9,10-linkage between the A and C-rings and extended the C4-chain on the A-ring in a stereoselective fashion. After derivatization of 16b into 15, the remaining 7-membered B-ring was cyclized in the presence of n-Bu3SnH and V-40 by utilizing the xanthate on the C-ring as the radical precursor and the allylic dithiocarbonate as the terminator. The second radical reaction thus enabled not only the 7-endo cyclization but also construction of the C8-stereocenter and the C6-exo olefin. Tricycle 14 was elaborated into the targeted 1 by a series of highly optimized chemoselective reactions. The present total synthesis of 1 demonstrates the advantages of radical reactions for linking hindered bonds within carbocycles without damaging preexisting functionalities, thereby offering a new strategic design for multistep target-oriented synthesis.
中文翻译:

通过自由基介导的三组分偶联和 7-内环化实现树脂毒素的全合成
Resiniferatoxin (1) 属于 daphnane diterpenoid 家族,对 TRPV1 具有强烈的激动作用,TRPV1 是一种有害温度和化学刺激的传感器。1 的高密度氧化反式融合 5/7/6-三碳环(ABC 环)对化学合成提出了艰巨的挑战。在这里,我们报告了一种新的基于自由基的策略的开发,用于从三个组件组装 1:A 环 9、烯丙基锡烷 18b 和 C 环 17b。由 d-核糖衍生物 19 制备的 6 元 17b 被设计为具有笼状原酸酯结构,α-烷氧基硒化物作为自由基前体。用 18b、9 和 V-40 处理 17b 后,从 17b 生成了具有强反应性的 α-烷氧基桥头自由基,然后依次与 9 和 18b 偶联得到 16b。第一个自由基反应形成了受阻的 C9,A 环和 C 环之间的 10-连接并以立体选择性方式扩展 A 环上的 C4 链。在将 16b 衍生为 15 后,剩余的 7 元 B 环在 n-Bu3SnH 和 V-40 存在下通过利用 C 环上的黄原酸酯作为自由基前体和烯丙基二硫代碳酸酯作为终止剂进行环化。因此,第二个自由基反应不仅能够进行 7-内环化,而且能够构建 C8-立体中心和 C6-外烯烃。通过一系列高度优化的化学选择性反应,三轮车 14 被加工成目标 1。目前 1 的全合成证明了自由基反应在不破坏预先存在的功能的情况下连接碳环内受阻键的优势,从而为多步靶向合成提供了新的战略设计。
更新日期:2017-10-31
中文翻译:

通过自由基介导的三组分偶联和 7-内环化实现树脂毒素的全合成
Resiniferatoxin (1) 属于 daphnane diterpenoid 家族,对 TRPV1 具有强烈的激动作用,TRPV1 是一种有害温度和化学刺激的传感器。1 的高密度氧化反式融合 5/7/6-三碳环(ABC 环)对化学合成提出了艰巨的挑战。在这里,我们报告了一种新的基于自由基的策略的开发,用于从三个组件组装 1:A 环 9、烯丙基锡烷 18b 和 C 环 17b。由 d-核糖衍生物 19 制备的 6 元 17b 被设计为具有笼状原酸酯结构,α-烷氧基硒化物作为自由基前体。用 18b、9 和 V-40 处理 17b 后,从 17b 生成了具有强反应性的 α-烷氧基桥头自由基,然后依次与 9 和 18b 偶联得到 16b。第一个自由基反应形成了受阻的 C9,A 环和 C 环之间的 10-连接并以立体选择性方式扩展 A 环上的 C4 链。在将 16b 衍生为 15 后,剩余的 7 元 B 环在 n-Bu3SnH 和 V-40 存在下通过利用 C 环上的黄原酸酯作为自由基前体和烯丙基二硫代碳酸酯作为终止剂进行环化。因此,第二个自由基反应不仅能够进行 7-内环化,而且能够构建 C8-立体中心和 C6-外烯烃。通过一系列高度优化的化学选择性反应,三轮车 14 被加工成目标 1。目前 1 的全合成证明了自由基反应在不破坏预先存在的功能的情况下连接碳环内受阻键的优势,从而为多步靶向合成提供了新的战略设计。































 京公网安备 11010802027423号
京公网安备 11010802027423号