当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
C3‐Symmetric Tricyclo[2.2.1.02,6]heptane‐3,5,7‐triol
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-11-03 , DOI: 10.1002/anie.201709279 Volodymyr Kozel 1 , Constantin-Gabriel Daniliuc 1 , Peer Kirsch 2 , Günter Haufe 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-11-03 , DOI: 10.1002/anie.201709279 Volodymyr Kozel 1 , Constantin-Gabriel Daniliuc 1 , Peer Kirsch 2 , Günter Haufe 1
Affiliation
|
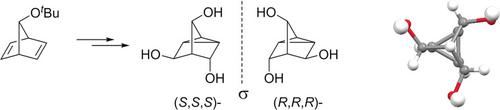
A straightforward access to a hitherto unknown C3‐symmetric tricyclic triol both in racemic and enantiopure forms has been developed. Treatment of 7‐tert‐butoxynorbornadiene with peroxycarboxylic acids provided mixtures of C1‐ and C3‐symmetric 3,5,7‐triacyloxynortricyclenes via transannular π‐cyclization and replacement of the tert‐butoxy group. By refluxing in formic acid, the C1‐symmetric esters were converted to the C3‐symmetric formate. Hydrolysis gave diastereoisomeric triols, which were separated by recrystallization. Enantiomer resolution via diastereoisomeric tri(O‐methylmandelates) delivered the target triols on a gram scale. The pure enantiomers are useful as core units of dopants for liquid crystals.
中文翻译:

C3对称三环[2.2.1.02,6]庚烷-3,5,7-三醇
已开发出一种可直接获得迄今未知的外消旋和对映纯形式的C 3对称三环三醇的方法。用过氧羧酸处理7-叔丁氧基降冰片二烯提供了C 1-和C 3-对称的3,5,7-三酰氧基降三环酮的混合物,通过环环π-环化和叔丁氧基取代。通过在甲酸中回流,C 1对称酯转化为C 3对称甲酸。水解得到非对映异构的三醇,将其通过重结晶分离。通过非对映异构体tri(O的对映体拆分甲基扁桃酸酯)以克为单位提供了目标三醇。纯对映体可用作液晶掺杂剂的核心单元。
更新日期:2017-11-03
中文翻译:

C3对称三环[2.2.1.02,6]庚烷-3,5,7-三醇
已开发出一种可直接获得迄今未知的外消旋和对映纯形式的C 3对称三环三醇的方法。用过氧羧酸处理7-叔丁氧基降冰片二烯提供了C 1-和C 3-对称的3,5,7-三酰氧基降三环酮的混合物,通过环环π-环化和叔丁氧基取代。通过在甲酸中回流,C 1对称酯转化为C 3对称甲酸。水解得到非对映异构的三醇,将其通过重结晶分离。通过非对映异构体tri(O的对映体拆分甲基扁桃酸酯)以克为单位提供了目标三醇。纯对映体可用作液晶掺杂剂的核心单元。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号