当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Three-dimensional context rather than NLS amino acid sequence determines importin α subtype specificity for RCC1.
Nature Communications ( IF 14.7 ) Pub Date : 2017-10-17 , DOI: 10.1038/s41467-017-01057-7 Rajeshwer S. Sankhala , Ravi K. Lokareddy , Salma Begum , Ruth A. Pumroy , Richard E. Gillilan , Gino Cingolani
Nature Communications ( IF 14.7 ) Pub Date : 2017-10-17 , DOI: 10.1038/s41467-017-01057-7 Rajeshwer S. Sankhala , Ravi K. Lokareddy , Salma Begum , Ruth A. Pumroy , Richard E. Gillilan , Gino Cingolani
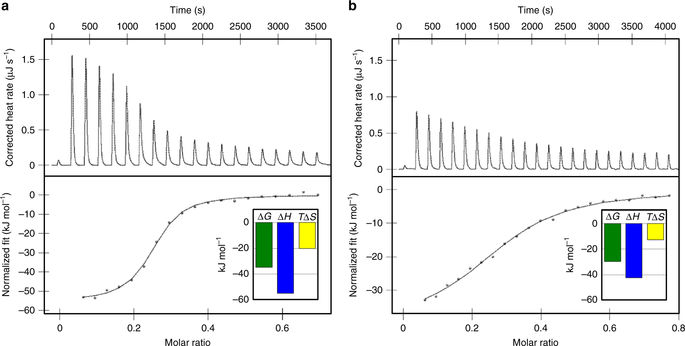
|
Active nuclear import of Ran exchange factor RCC1 is mediated by importin α3. This pathway is essential to generate a gradient of RanGTP on chromatin that directs nucleocytoplasmic transport, mitotic spindle assembly and nuclear envelope formation. Here we identify the mechanisms of importin α3 selectivity for RCC1. We find this isoform binds RCC1 with one order of magnitude higher affinity than the generic importin α1, although the two isoforms share an identical NLS-binding groove. Importin α3 uses its greater conformational flexibility to wedge the RCC1 β-propeller flanking the NLS against its lateral surface, preventing steric clashes with its Armadillo-core. Removing the β-propeller, or inserting a linker between NLS and β-propeller, disrupts specificity for importin α3, demonstrating the structural context rather than NLS sequence determines selectivity for isoform 3. We propose importin α3 evolved to recognize topologically complex NLSs that lie next to bulky domains or are masked by quaternary structures.Importin α3 facilitates the nuclear transport of the Ran guanine nucleotide exchange factor RCC1. Here the authors reveal the molecular basis for the selectivity of RCC1 for importin α3 vs the generic importin α1 and discuss the evolution of importin α isoforms.
中文翻译:

三维背景而非NLS氨基酸序列决定了RCC1的重要α亚型特异性。
Ran交换因子RCC1的活性核输入是由importinα3介导的。该途径对于在染色质上产生RanGTP梯度至关重要,该梯度可指导核质运输,有丝分裂纺锤体组装和核包膜形成。在这里,我们确定了RCC1的重要性α3选择性的机制。我们发现这种同工型与RCC1的亲和力比通用导入素α1高一个数量级,尽管这两个同工型共享相同的NLS结合槽。Importinα3利用其更大的构象柔韧性将NLS侧面的RCC1β螺旋桨楔入其侧面,从而防止其犰狳核心发生空间冲突。移除β螺旋桨或在NLS和β螺旋桨之间插入连接子会破坏输入蛋白α3的特异性,证明结构上下文而不是NLS序列决定了同工型3的选择性。我们提出importinα3的发展是为了识别拓扑结构复杂的NLS,其位于庞大的结构域附近或被四级结构所掩盖。Importinα3促进了Ran鸟嘌呤核苷酸交换因子的核转运。 RCC1。在这里,作者揭示了RCC1对importinα3相对于一般importinα1的选择性的分子基础,并讨论了importinα同工型的演变。
更新日期:2017-10-17
中文翻译:

三维背景而非NLS氨基酸序列决定了RCC1的重要α亚型特异性。
Ran交换因子RCC1的活性核输入是由importinα3介导的。该途径对于在染色质上产生RanGTP梯度至关重要,该梯度可指导核质运输,有丝分裂纺锤体组装和核包膜形成。在这里,我们确定了RCC1的重要性α3选择性的机制。我们发现这种同工型与RCC1的亲和力比通用导入素α1高一个数量级,尽管这两个同工型共享相同的NLS结合槽。Importinα3利用其更大的构象柔韧性将NLS侧面的RCC1β螺旋桨楔入其侧面,从而防止其犰狳核心发生空间冲突。移除β螺旋桨或在NLS和β螺旋桨之间插入连接子会破坏输入蛋白α3的特异性,证明结构上下文而不是NLS序列决定了同工型3的选择性。我们提出importinα3的发展是为了识别拓扑结构复杂的NLS,其位于庞大的结构域附近或被四级结构所掩盖。Importinα3促进了Ran鸟嘌呤核苷酸交换因子的核转运。 RCC1。在这里,作者揭示了RCC1对importinα3相对于一般importinα1的选择性的分子基础,并讨论了importinα同工型的演变。

































 京公网安备 11010802027423号
京公网安备 11010802027423号