Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Oral Semaglutide Compared With Placebo and Subcutaneous Semaglutide on Glycemic Control in Patients With Type 2 Diabetes
JAMA ( IF 63.1 ) Pub Date : 2017-10-17 , DOI: 10.1001/jama.2017.14752 Melanie Davies 1 , Thomas R. Pieber 2 , Marie-Louise Hartoft-Nielsen 3 , Oluf K. H. Hansen 3 , Serge Jabbour 4 , Julio Rosenstock 5
JAMA ( IF 63.1 ) Pub Date : 2017-10-17 , DOI: 10.1001/jama.2017.14752 Melanie Davies 1 , Thomas R. Pieber 2 , Marie-Louise Hartoft-Nielsen 3 , Oluf K. H. Hansen 3 , Serge Jabbour 4 , Julio Rosenstock 5
Affiliation
|
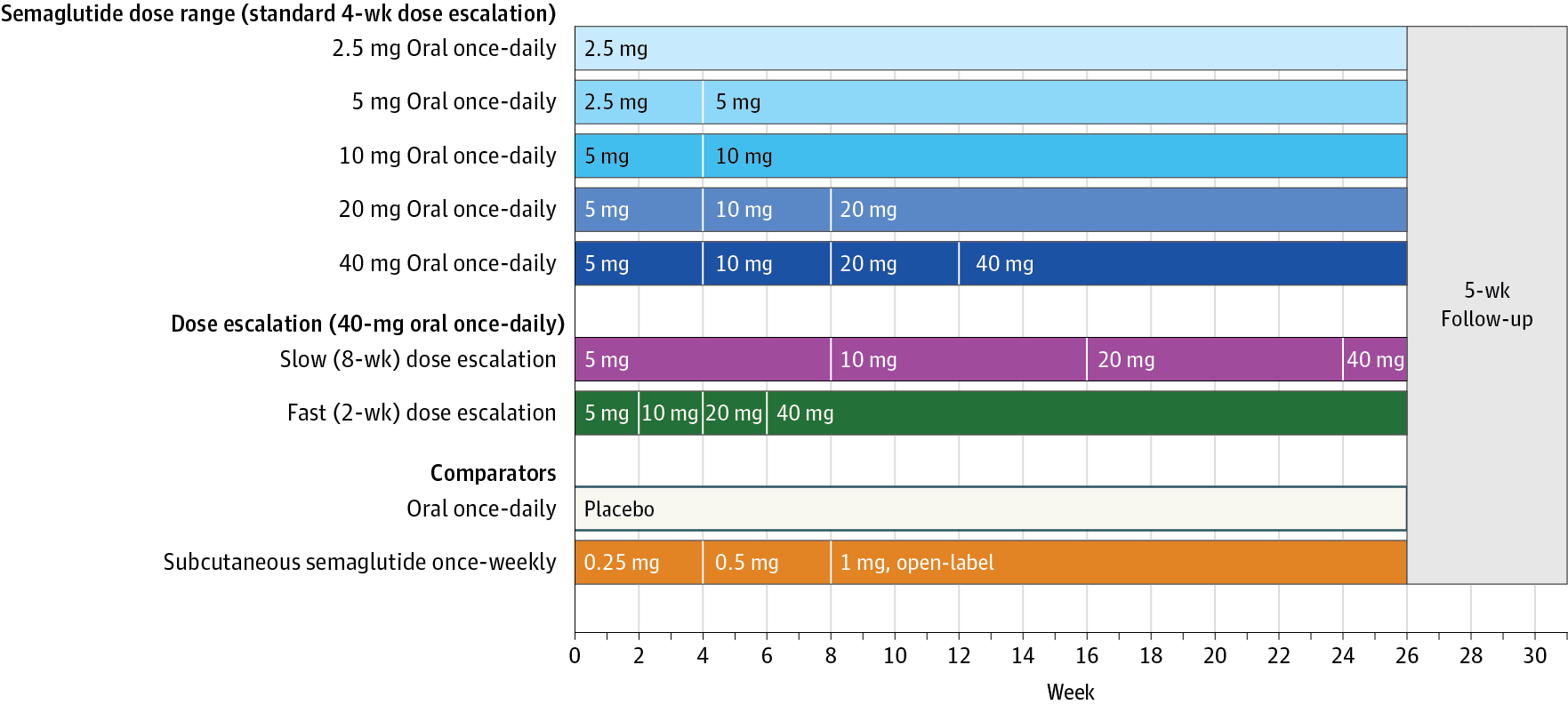
Importance Glucagon-like peptide-1 (GLP-1) receptor agonists are effective therapies for the treatment of type 2 diabetes and are all currently available as an injection. Objectives To compare the effects of oral semaglutide with placebo (primary) and open-label subcutaneous semaglutide (secondary) on glycemic control in patients with type 2 diabetes. Design, Setting, and Patients Phase 2, randomized, parallel-group, dosage-finding, 26-week trial with 5-week follow-up at 100 sites (hospital clinics, general practices, and clinical research centers) in 14 countries conducted between December 2013 and December 2014. Of 1106 participants assessed, 632 with type 2 diabetes and insufficient glycemic control using diet and exercise alone or a stable dose of metformin were randomized. Randomization was stratified by metformin use. Interventions Once-daily oral semaglutide of 2.5 mg (n = 70), 5 mg (n = 70), 10 mg (n = 70), 20 mg (n = 70), 40-mg 4-week dose escalation (standard escalation; n = 71), 40-mg 8-week dose escalation (slow escalation; n = 70), 40-mg 2-week dose escalation (fast escalation, n = 70), oral placebo (n = 71; double-blind) or once-weekly subcutaneous semaglutide of 1.0 mg (n = 70) for 26 weeks. Main Outcomes and Measures The primary end point was change in hemoglobing A1c (HbA1c) from baseline to week 26. Secondary end points included change from baseline in body weight and adverse events. Results Baseline characteristics were comparable across treatment groups. Of the 632 randomized patients (mean age, 57.1 years [SD, 10.6]; men, 395 (62.7%); diabetes duration, 6.3 years [SD, 5.2]; body weight, 92.3 kg [SD, 16.8]; BMI, 31.7 [SD, 4.3]), 583 (92%) completed the trial. Mean change in HbA1c level from baseline to week 26 decreased with oral semaglutide (dosage-dependent range, −0.7% to −1.9%) and subcutaneous semaglutide (−1.9%) and placebo (−0.3%); oral semaglutide reductions were significant vs placebo (dosage-dependent estimated treatment difference [ETD] range for oral semaglutide vs placebo, –0.4% to –1.6%; P = .01 for 2.5 mg, <.001 for all other dosages). Reductions in body weight were greater with oral semaglutide (dosage-dependent range, −2.1 kg to −6.9 kg) and subcutaneous semaglutide (−6.4 kg) vs placebo (−1.2 kg), and significant for oral semaglutide dosages of 10 mg or more vs placebo (dosage-dependent ETD range, –0.9 to –5.7 kg; P < .001). Adverse events were reported by 63% to 86% (371 of 490 patients) in the oral semaglutide groups, 81% (56 of 69 patients) in the subcutaneous semaglutide group, and 68% (48 of 71 patients) in the placebo group; mild to moderate gastrointestinal events were most common. Conclusions and Relevance Among patients with type 2 diabetes, oral semaglutide resulted in better glycemic control than placebo over 26 weeks. These findings support phase 3 studies to assess longer-term and clinical outcomes, as well as safety. Trial Registration clinicaltrials.gov Identifier: NCT01923181
中文翻译:

口服司美鲁肽与安慰剂和皮下司美鲁肽相比对 2 型糖尿病患者血糖控制的影响
重要性 胰高血糖素样肽-1 (GLP-1) 受体激动剂是治疗 2 型糖尿病的有效疗法,目前均可作为注射剂使用。目的 比较口服司美鲁肽与安慰剂(主要)和开放标签皮下注射司美鲁肽(次要)对 2 型糖尿病患者血糖控制的影响。设计、设置和患者 2 期、随机、平行组、剂量探索、26 周试验,在 14 个国家的 100 个地点(医院诊所、全科诊所和临床研究中心)进行了 5 周的随访2013 年 12 月和 2014 年 12 月。在评估的 1106 名参与者中,632 名患有 2 型糖尿病且仅使用饮食和运动或稳定剂量的二甲双胍控制血糖不足的参与者被随机分组。通过二甲双胍的使用对随机化进行分层。干预 每日一次口服司美鲁肽 2.5 mg (n = 70)、5 mg (n = 70)、10 mg (n = 70)、20 mg (n = 70)、40 mg 4 周剂量递增(标准递增) ;n = 71),40 毫克 8 周剂量递增(缓慢递增;n = 70),40 毫克 2 周剂量递增(快速递增,n = 70),口服安慰剂(n = 71;双盲) ) 或每周一次皮下注射 1.0 mg (n = 70) 的司美鲁肽,共 26 周。主要结果和测量主要终点是从基线到第 26 周血红蛋白 A1c (HbA1c) 的变化。次要终点包括体重和不良事件相对于基线的变化。结果 各治疗组的基线特征具有可比性。在 632 名随机患者中(平均年龄,57.1 岁 [SD,10.6];男性,395 (62.7%);糖尿病病程,6.3 年 [SD,5.2];体重,92.3 kg [SD,16.8];BMI,31.7) [SD, 4.3]), 583 人(92%)完成了试验。HbA1c 水平从基线到第 26 周的平均变化随着口服司美鲁肽(剂量依赖性范围,-0.7% 至 -1.9%)和皮下司美鲁肽(-1.9%)和安慰剂(-0.3%)而降低;口服司美鲁肽与安慰剂相比显着减少(口服司美鲁肽与安慰剂的剂量依赖性估计治疗差异 [ETD] 范围,–0.4% 至 –1.6%;2.5 mg 的 P = .01,所有其他剂量的 P = .001)。与安慰剂(-1.2 kg)相比,口服司美鲁肽(剂量依赖性范围,-2.1 kg 至 -6.9 kg)和皮下注射司美鲁肽(-6.4 kg)对体重的减轻更大,并且对于 10 mg 或更多的口服司美鲁肽剂量显着对比安慰剂(剂量依赖性 ETD 范围,–0.9 至 –5.7 kg;P < .001)。在口服司美鲁肽组中,63% 至 86%(490 名患者中的 371 名)报告了不良事件,皮下注射司美鲁肽组为 81%(69 名患者中的 56 名),安慰剂组为 68%(71 名患者中的 48 名);轻度至中度胃肠道事件最常见。结论和相关性 在 2 型糖尿病患者中,口服司美鲁肽在 26 周内比安慰剂更好地控制了血糖。这些发现支持 3 期研究,以评估长期和临床结果以及安全性。试验注册clinicaltrials.gov 标识符:NCT01923181 以及安全。试验注册clinicaltrials.gov 标识符:NCT01923181 以及安全。试验注册clinicaltrials.gov 标识符:NCT01923181
更新日期:2017-10-17
中文翻译:

口服司美鲁肽与安慰剂和皮下司美鲁肽相比对 2 型糖尿病患者血糖控制的影响
重要性 胰高血糖素样肽-1 (GLP-1) 受体激动剂是治疗 2 型糖尿病的有效疗法,目前均可作为注射剂使用。目的 比较口服司美鲁肽与安慰剂(主要)和开放标签皮下注射司美鲁肽(次要)对 2 型糖尿病患者血糖控制的影响。设计、设置和患者 2 期、随机、平行组、剂量探索、26 周试验,在 14 个国家的 100 个地点(医院诊所、全科诊所和临床研究中心)进行了 5 周的随访2013 年 12 月和 2014 年 12 月。在评估的 1106 名参与者中,632 名患有 2 型糖尿病且仅使用饮食和运动或稳定剂量的二甲双胍控制血糖不足的参与者被随机分组。通过二甲双胍的使用对随机化进行分层。干预 每日一次口服司美鲁肽 2.5 mg (n = 70)、5 mg (n = 70)、10 mg (n = 70)、20 mg (n = 70)、40 mg 4 周剂量递增(标准递增) ;n = 71),40 毫克 8 周剂量递增(缓慢递增;n = 70),40 毫克 2 周剂量递增(快速递增,n = 70),口服安慰剂(n = 71;双盲) ) 或每周一次皮下注射 1.0 mg (n = 70) 的司美鲁肽,共 26 周。主要结果和测量主要终点是从基线到第 26 周血红蛋白 A1c (HbA1c) 的变化。次要终点包括体重和不良事件相对于基线的变化。结果 各治疗组的基线特征具有可比性。在 632 名随机患者中(平均年龄,57.1 岁 [SD,10.6];男性,395 (62.7%);糖尿病病程,6.3 年 [SD,5.2];体重,92.3 kg [SD,16.8];BMI,31.7) [SD, 4.3]), 583 人(92%)完成了试验。HbA1c 水平从基线到第 26 周的平均变化随着口服司美鲁肽(剂量依赖性范围,-0.7% 至 -1.9%)和皮下司美鲁肽(-1.9%)和安慰剂(-0.3%)而降低;口服司美鲁肽与安慰剂相比显着减少(口服司美鲁肽与安慰剂的剂量依赖性估计治疗差异 [ETD] 范围,–0.4% 至 –1.6%;2.5 mg 的 P = .01,所有其他剂量的 P = .001)。与安慰剂(-1.2 kg)相比,口服司美鲁肽(剂量依赖性范围,-2.1 kg 至 -6.9 kg)和皮下注射司美鲁肽(-6.4 kg)对体重的减轻更大,并且对于 10 mg 或更多的口服司美鲁肽剂量显着对比安慰剂(剂量依赖性 ETD 范围,–0.9 至 –5.7 kg;P < .001)。在口服司美鲁肽组中,63% 至 86%(490 名患者中的 371 名)报告了不良事件,皮下注射司美鲁肽组为 81%(69 名患者中的 56 名),安慰剂组为 68%(71 名患者中的 48 名);轻度至中度胃肠道事件最常见。结论和相关性 在 2 型糖尿病患者中,口服司美鲁肽在 26 周内比安慰剂更好地控制了血糖。这些发现支持 3 期研究,以评估长期和临床结果以及安全性。试验注册clinicaltrials.gov 标识符:NCT01923181 以及安全。试验注册clinicaltrials.gov 标识符:NCT01923181 以及安全。试验注册clinicaltrials.gov 标识符:NCT01923181






























 京公网安备 11010802027423号
京公网安备 11010802027423号