当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Designer α1,6-Fucosidase Mutants Enable Direct Core Fucosylation of Intact N-Glycopeptides and N-Glycoproteins
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-10-16 00:00:00 , DOI: 10.1021/jacs.7b07906
Chao Li 1 , Shilei Zhu 1 , Christopher Ma 1 , Lai-Xi Wang 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-10-16 00:00:00 , DOI: 10.1021/jacs.7b07906
Chao Li 1 , Shilei Zhu 1 , Christopher Ma 1 , Lai-Xi Wang 1
Affiliation
|
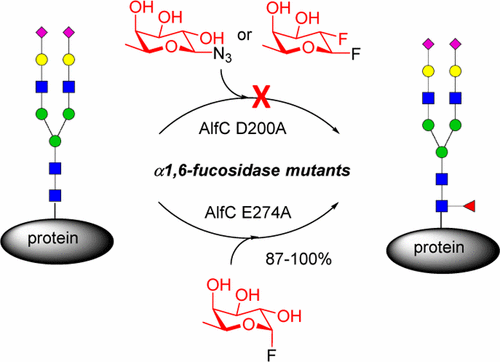
Core fucosylation of N-glycoproteins plays a crucial role in modulating the biological functions of glycoproteins. Yet, the synthesis of structurally well-defined, core-fucosylated glycoproteins remains a challenging task due to the complexity in multistep chemical synthesis or the inability of the biosynthetic α1,6-fucosyltransferase (FUT8) to directly fucosylate full-size mature N-glycans in a chemoenzymatic approach. We report in this paper the design and generation of potential α1,6-fucosynthase and fucoligase for direct core fucosylation of intact N-glycoproteins. We found that mutation at the nucleophilic residue (D200) did not provide a typical glycosynthase from this bacterial enzyme, but several mutants with mutation at the general acid/base residue E274 of the Lactobacillus casei α1,6-fucosidase, including E274A, E274S, and E274G, acted as efficient glycoligases that could fucosylate a wide variety of complex N-glycopeptides and intact glycoproteins by using α-fucosyl fluoride as a simple donor substrate. Studies on the substrate specificity revealed that the α1,6-fucosidase mutants could introduce an α1,6-fucose moiety specifically at the Asn-linked GlcNAc moiety not only to GlcNAc-peptide but also to high-mannose and complex-type N-glycans in the context of N-glycopeptides, N-glycoproteins, and intact antibodies. This discovery opens a new avenue to a wide variety of homogeneous, core-fucosylated N-glycopeptides and N-glycoproteins that are hitherto difficult to obtain for structural and functional studies.
中文翻译:

Designerα1,6-岩藻糖苷酶突变体可实现完整N-糖肽和N-糖蛋白的直接核心岩藻糖基化
N-糖蛋白的核心岩藻糖基化在调节糖蛋白的生物学功能中起着至关重要的作用。然而,由于多步化学合成的复杂性或生物合成α1,6-岩藻糖基转移酶(FUT8)无法直接岩藻糖化全尺寸成熟N-聚糖,结构明确的核心岩藻糖基化糖蛋白的合成仍然是一项艰巨的任务。在化学酶学方法中。我们在本文中报道了完整的N-糖蛋白直接核心岩藻糖基化的潜在α1,6-岩藻糖合酶和岩藻糖酶的设计和生成。我们发现亲核残基(D200)上的突变并未从该细菌酶中提供典型的糖合酶,而是几个干酪乳杆菌的一般酸/碱残基E274处具有突变的突变体α1,6-岩藻糖苷酶(包括E274A,E274S和E274G)可作为有效的糖基化酶,通过使用α-岩藻糖基氟化物作为简单的供体底物,可以岩藻糖基化多种复杂的N-糖肽和完整的糖蛋白。对底物特异性的研究表明,α1,6-岩藻糖苷酶突变体不仅可以在Asn连接的GlcNAc部分上引入一个α1,6-岩藻糖部分,不仅对GlcNAc肽而且对高甘露糖和复杂型N-聚糖都具有影响。在N-糖肽,N-糖蛋白和完整抗体的情况下。这一发现为迄今难以通过结构和功能研究获得的各种均质的,核心岩藻糖基化的N-糖肽和N-糖蛋白开辟了一条新途径。
更新日期:2017-10-16
中文翻译:

Designerα1,6-岩藻糖苷酶突变体可实现完整N-糖肽和N-糖蛋白的直接核心岩藻糖基化
N-糖蛋白的核心岩藻糖基化在调节糖蛋白的生物学功能中起着至关重要的作用。然而,由于多步化学合成的复杂性或生物合成α1,6-岩藻糖基转移酶(FUT8)无法直接岩藻糖化全尺寸成熟N-聚糖,结构明确的核心岩藻糖基化糖蛋白的合成仍然是一项艰巨的任务。在化学酶学方法中。我们在本文中报道了完整的N-糖蛋白直接核心岩藻糖基化的潜在α1,6-岩藻糖合酶和岩藻糖酶的设计和生成。我们发现亲核残基(D200)上的突变并未从该细菌酶中提供典型的糖合酶,而是几个干酪乳杆菌的一般酸/碱残基E274处具有突变的突变体α1,6-岩藻糖苷酶(包括E274A,E274S和E274G)可作为有效的糖基化酶,通过使用α-岩藻糖基氟化物作为简单的供体底物,可以岩藻糖基化多种复杂的N-糖肽和完整的糖蛋白。对底物特异性的研究表明,α1,6-岩藻糖苷酶突变体不仅可以在Asn连接的GlcNAc部分上引入一个α1,6-岩藻糖部分,不仅对GlcNAc肽而且对高甘露糖和复杂型N-聚糖都具有影响。在N-糖肽,N-糖蛋白和完整抗体的情况下。这一发现为迄今难以通过结构和功能研究获得的各种均质的,核心岩藻糖基化的N-糖肽和N-糖蛋白开辟了一条新途径。

































 京公网安备 11010802027423号
京公网安备 11010802027423号