当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Activation of Epoxides by a Cooperative Iron–Thiolate Catalyst: Intermediacy of Ferrous Alkoxides in Catalytic Hydroboration
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-10-13 00:00:00 , DOI: 10.1021/acscatal.7b02527 Heng Song 1 , Ke Ye 2 , Peiyu Geng 1 , Xiao Han 1 , Rongzhen Liao 2 , Chen-Ho Tung 1 , Wenguang Wang 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-10-13 00:00:00 , DOI: 10.1021/acscatal.7b02527 Heng Song 1 , Ke Ye 2 , Peiyu Geng 1 , Xiao Han 1 , Rongzhen Liao 2 , Chen-Ho Tung 1 , Wenguang Wang 1
Affiliation
|
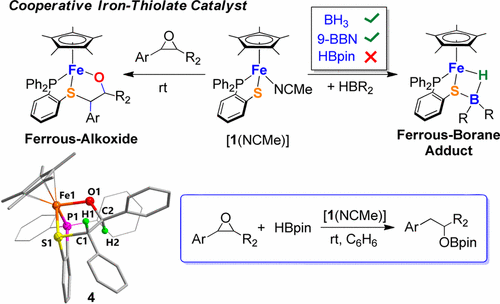
This paper describes a cooperative iron–thiolate catalyst Cp*Fe(1,2-Ph2PC6H4S)(NCMe) (Cp*– = C5Me5–, [1(NCMe)]) for regioselective hydroboration of aryl epoxide by pinacolborane (HBpin). The critical catalytic step involves the direct addition of epoxide to the catalyst rather than activation of the B–H bond of HBpin. Through iron–thiolate cooperation, [1(NCMe)] opens the aryl epoxide rings affording ferrous–alkoxide compounds. Notably, the ferrous–alkoxide intermediate (4) was structurally characterized after its isolation from the reaction of [1(NCMe)] with trans-2,3-diphenyloxirane. The more Lewis acidic hydroboranes such as H3B·THF and 9-BBN (BBN = borabicyclononane) can also be captured by [1(NCMe)]. The resulting iron–borane adducts [1H(BH2)] and [1H(BBN)] feature an agnostic Fe···B–H interaction. DFT calculations indicate that the addition of HBpin across the iron–thiolate sites is endergonic by 12.9 kcal/mol, whereas it is exergonic by 20.2 kcal/mol with BH3 and 4.6 kcal/mol with 9-BBN. Combining the experimental data with theoretical studies, a mechanism of the substrate activation by [1(NCMe)], followed by HBpin addition, is proposed for the catalysis.
中文翻译:

硫铁酸盐协同催化剂对环氧的活化:催化氢硼化反应中亚铁醇的中介作用
本文描述了一种协同硫代铁催化Cp * Fe(1,2-Ph 2 PC 6 H 4 S)(NCMe)(Cp * – = C 5 Me 5 –,[ 1(NCMe)])的区域选择性硼氢化反应。频哪醇硼烷(HBpin)形成芳基环氧化物。关键的催化步骤涉及直接向催化剂中添加环氧化物,而不是激活HBpin的B–H键。通过硫醇铁的合作,[ 1(NCMe)]打开芳基环氧环,提供亚铁醇盐化合物。值得注意的是,铁基醇盐中间体(4)在结构上表征其分离后从[反应1(NCMe)]与反式-2,3-二苯基环氧乙烷。[ 1(NCMe)]也可以捕获更多的路易斯酸性氢硼烷,例如H 3 B·THF和9-BBN(BBN =硼环环壬烷)。生成的铁硼烷加合物[ 1 H(BH 2)]和[ 1 H(BBN)]具有不可知的Fe···B–H相互作用。DFT计算表明,跨硫铁酸位点添加HBpin的透热性为12.9 kcal / mol,而对于BH 3为HBgpin为20.2 kcal / mol,对于9-BBN则为4.6 kcal / mol。结合实验数据和理论研究,提出了一种通过[ 1(NCMe)]活化底物,然后添加HBpin的催化机理。
更新日期:2017-10-13
中文翻译:

硫铁酸盐协同催化剂对环氧的活化:催化氢硼化反应中亚铁醇的中介作用
本文描述了一种协同硫代铁催化Cp * Fe(1,2-Ph 2 PC 6 H 4 S)(NCMe)(Cp * – = C 5 Me 5 –,[ 1(NCMe)])的区域选择性硼氢化反应。频哪醇硼烷(HBpin)形成芳基环氧化物。关键的催化步骤涉及直接向催化剂中添加环氧化物,而不是激活HBpin的B–H键。通过硫醇铁的合作,[ 1(NCMe)]打开芳基环氧环,提供亚铁醇盐化合物。值得注意的是,铁基醇盐中间体(4)在结构上表征其分离后从[反应1(NCMe)]与反式-2,3-二苯基环氧乙烷。[ 1(NCMe)]也可以捕获更多的路易斯酸性氢硼烷,例如H 3 B·THF和9-BBN(BBN =硼环环壬烷)。生成的铁硼烷加合物[ 1 H(BH 2)]和[ 1 H(BBN)]具有不可知的Fe···B–H相互作用。DFT计算表明,跨硫铁酸位点添加HBpin的透热性为12.9 kcal / mol,而对于BH 3为HBgpin为20.2 kcal / mol,对于9-BBN则为4.6 kcal / mol。结合实验数据和理论研究,提出了一种通过[ 1(NCMe)]活化底物,然后添加HBpin的催化机理。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号