当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Benzophenone Nucleosides and Their Photocatalytic Evaluation for [2+2] Cycloaddition in Aqueous Media
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2015-09-09 , DOI: 10.1002/ejoc.201500885
Nadine Gaß , Hans‐Achim Wagenknecht
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2015-09-09 , DOI: 10.1002/ejoc.201500885
Nadine Gaß , Hans‐Achim Wagenknecht
|
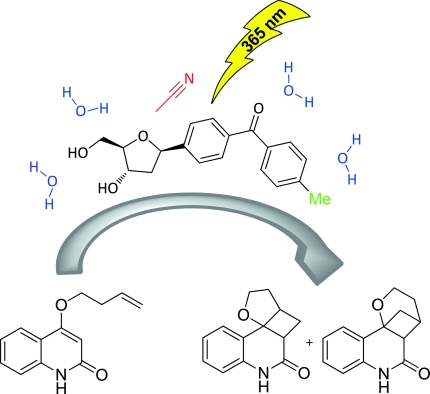
Four benzophenone nucleosides that are para-substituted (–NH2, –NMe2, –OMe, and –Me) in relation to the carbonyl group were synthesized and characterized by their optical properties. The electron-donating character of the substituents influenced the optical properties of these nucleosides, especially the bathochromic shift of the charge-transfer band in their UV/Vis absorption spectra. The solubility of the synthetic nucleosides in aqueous solution allowed for a photocatalytic intramolecular [2+2] cycloaddition of a quinolone substrate to take place in H2O/MeCN by irradiating the mixture with 365 nm light-emitting diode (LED) lamps. The MeO- and Me-substituted benzophenone nucleosides were subjected to these reaction conditions in substoichiometric amounts. After prolonged irradiation times, substrate conversions competed with product decomposition. On the basis of our results, we determined that the Me-substituted benzophenone nucleoside has potential applications in the development of photocatalytically active DNAzymes for both in vivo applications in chemical biology and enantioselective photocatalysis in aqueous solutions.
中文翻译:

二苯甲酮核苷的合成及其对水性介质中[2+2]环加成的光催化评价
合成了四种与羰基相关的对位取代的二苯甲酮核苷(–NH2、–NMe2、–OMe 和 –Me),并通过它们的光学性质进行表征。取代基的给电子特性影响了这些核苷的光学性质,尤其是其紫外/可见吸收光谱中电荷转移带的红移。合成核苷在水溶液中的溶解度允许通过用 365 nm 发光二极管 (LED) 灯照射混合物,在 H2O/MeCN 中发生喹诺酮底物的光催化分子内 [2+2] 环加成。MeO-和Me-取代的二苯甲酮核苷以亚化学计量的量经受这些反应条件。经过长时间的照射,底物转化与产物分解竞争。基于我们的研究结果,我们确定 Me 取代的二苯甲酮核苷在开发具有光催化活性的 DNA 酶方面具有潜在的应用价值,可用于化学生物学的体内应用和水溶液中的对映选择性光催化。
更新日期:2015-09-09
中文翻译:

二苯甲酮核苷的合成及其对水性介质中[2+2]环加成的光催化评价
合成了四种与羰基相关的对位取代的二苯甲酮核苷(–NH2、–NMe2、–OMe 和 –Me),并通过它们的光学性质进行表征。取代基的给电子特性影响了这些核苷的光学性质,尤其是其紫外/可见吸收光谱中电荷转移带的红移。合成核苷在水溶液中的溶解度允许通过用 365 nm 发光二极管 (LED) 灯照射混合物,在 H2O/MeCN 中发生喹诺酮底物的光催化分子内 [2+2] 环加成。MeO-和Me-取代的二苯甲酮核苷以亚化学计量的量经受这些反应条件。经过长时间的照射,底物转化与产物分解竞争。基于我们的研究结果,我们确定 Me 取代的二苯甲酮核苷在开发具有光催化活性的 DNA 酶方面具有潜在的应用价值,可用于化学生物学的体内应用和水溶液中的对映选择性光催化。




































 京公网安备 11010802027423号
京公网安备 11010802027423号