当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Concise Synthesis to Benzo[c]cinnolines via 2,2’‐Dinitro‐1,1’‐Biphenyls Attained from a Novel Tailored Suzuki Cross‐Coupling
ChemistrySelect ( IF 1.9 ) Pub Date : 2017-10-12 , DOI: 10.1002/slct.201701993 Vijayaragavan Elumalai 1 , Hans-René Bjørsvik 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2017-10-12 , DOI: 10.1002/slct.201701993 Vijayaragavan Elumalai 1 , Hans-René Bjørsvik 1
Affiliation
|
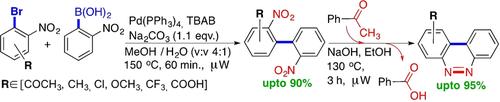
A new two‐step synthetic process for the preparation of unsymmetrically substituted benzo[c]cinnolines was developed. The key intermediate 2,2’‐dinitro‐1,1’‐biphenyl was prepared by means of an unprecedented tailored Suzuki cross‐coupling protocol. The subsequent step is constituted by a domino partial nitro group reduction and intramolecular diazo bond formation, a process that afford target benzo[c]cinnoline scaffold. The disclosed method tolerates both electron‐withdrawing‐ and electron‐donating groups.
中文翻译:

通过新型量身定制的铃木交叉偶联反应通过2,2'-二硝基-1,1'-联苯合成苯并[c] cinnolines的简明方法
开发了一种新的两步合成方法,用于制备不对称取代的苯并[ c ]肉桂醛。关键的中间体2,2'-二硝基-1,1'-联苯是通过前所未有的量身定制的Suzuki交叉偶联方案制备的。后续步骤由多米诺骨牌部分硝基还原和分子内重氮键形成组成,该过程提供了目标苯并[ c ]肉桂酸支架。所公开的方法容许吸电子基团和给电子基团。
更新日期:2017-10-12
中文翻译:

通过新型量身定制的铃木交叉偶联反应通过2,2'-二硝基-1,1'-联苯合成苯并[c] cinnolines的简明方法
开发了一种新的两步合成方法,用于制备不对称取代的苯并[ c ]肉桂醛。关键的中间体2,2'-二硝基-1,1'-联苯是通过前所未有的量身定制的Suzuki交叉偶联方案制备的。后续步骤由多米诺骨牌部分硝基还原和分子内重氮键形成组成,该过程提供了目标苯并[ c ]肉桂酸支架。所公开的方法容许吸电子基团和给电子基团。





































 京公网安备 11010802027423号
京公网安备 11010802027423号