当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of N-(4-(2,4-Difluorophenoxy)-3-(6-methyl-7-oxo-6,7-dihydro-1H-pyrrolo[2,3-c]pyridin-4-yl)phenyl)ethanesulfonamide (ABBV-075/Mivebresib), a Potent and Orally Available Bromodomain and Extraterminal Domain (BET) Family Bromodomain Inhibitor
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2017-10-12 00:00:00 , DOI: 10.1021/acs.jmedchem.7b00746 Keith F. McDaniel 1 , Le Wang 1 , Todd Soltwedel 1 , Steven D. Fidanze 1 , Lisa A. Hasvold 1 , Dachun Liu 1 , Robert A. Mantei 1 , John K. Pratt 1 , George S. Sheppard 1 , Mai H. Bui 1 , Emily J. Faivre 1 , Xiaoli Huang 1 , Leiming Li 1 , Xiaoyu Lin 1 , Rongqi Wang 1 , Scott E. Warder 1 , Denise Wilcox 1 , Daniel H. Albert 1 , Terrance J. Magoc 1 , Ganesh Rajaraman 1 , Chang H. Park 1 , Charles W. Hutchins 1 , Jianwei J. Shen 1 , Rohinton P. Edalji 1 , Chaohong C. Sun 1 , Ruth Martin 1 , Wenqing Gao 1 , Shekman Wong 1 , Guowei Fang 1 , Steven W. Elmore 1 , Yu Shen 1 , Warren M. Kati 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2017-10-12 00:00:00 , DOI: 10.1021/acs.jmedchem.7b00746 Keith F. McDaniel 1 , Le Wang 1 , Todd Soltwedel 1 , Steven D. Fidanze 1 , Lisa A. Hasvold 1 , Dachun Liu 1 , Robert A. Mantei 1 , John K. Pratt 1 , George S. Sheppard 1 , Mai H. Bui 1 , Emily J. Faivre 1 , Xiaoli Huang 1 , Leiming Li 1 , Xiaoyu Lin 1 , Rongqi Wang 1 , Scott E. Warder 1 , Denise Wilcox 1 , Daniel H. Albert 1 , Terrance J. Magoc 1 , Ganesh Rajaraman 1 , Chang H. Park 1 , Charles W. Hutchins 1 , Jianwei J. Shen 1 , Rohinton P. Edalji 1 , Chaohong C. Sun 1 , Ruth Martin 1 , Wenqing Gao 1 , Shekman Wong 1 , Guowei Fang 1 , Steven W. Elmore 1 , Yu Shen 1 , Warren M. Kati 1
Affiliation
|
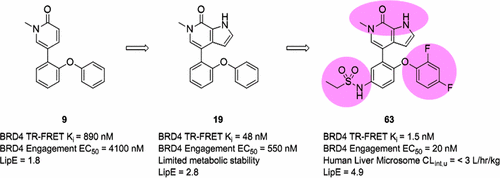
The development of bromodomain and extraterminal domain (BET) bromodomain inhibitors and their examination in clinical studies, particularly in oncology settings, has garnered substantial recent interest. An effort to generate novel BET bromodomain inhibitors with excellent potency and drug metabolism and pharmacokinetics (DMPK) properties was initiated based upon elaboration of a simple pyridone core. Efforts to develop a bidentate interaction with a critical asparagine residue resulted in the incorporation of a pyrrolopyridone core, which improved potency by 9–19-fold. Additional structure–activity relationship (SAR) efforts aimed both at increasing potency and improving pharmacokinetic properties led to the discovery of the clinical candidate 63 (ABBV-075/mivebresib), which demonstrates excellent potency in biochemical and cellular assays, advantageous exposures and half-life both in animal models and in humans, and in vivo efficacy in mouse models of cancer progression and inflammation.
中文翻译:

的发现ñ - (4-(2,4-二氟苯氧基)-3-(6-甲基-7-氧代-6,7-二氢- 1 H ^ -吡咯并[2,3- c ^ ]吡啶-4-基)苯基)乙磺酰胺(ABBV-075 / Mivebresib),有效且可口服的Bromodomain和Extraterminal Domain(BET)家族Bromodomain抑制剂
溴结构域和末端外结构域(BET)溴结构域抑制剂的开发及其在临床研究中,特别是在肿瘤学领域中的检查,引起了人们的广泛关注。基于简单的吡啶酮核心的制备,人们开始努力产生具有出色效能,药物代谢和药代动力学(DMPK)特性的新型BET溴结构域抑制剂。与关键的天冬酰胺残基形成双齿相互作用的努力导致掺入了吡咯并吡啶酮核心,其效价提高了9-19倍。旨在提高效力和改善药代动力学特性的额外结构-活性关系(SAR)努力导致了临床候选药物的发现63 (ABBV-075 / mivebresib),其在生化和细胞分析中显示出优异的效力,在动物模型和人类模型中均具有良好的暴露效果和半衰期,并且在癌症进展和炎症的小鼠模型中具有体内功效。
更新日期:2017-10-12
中文翻译:

的发现ñ - (4-(2,4-二氟苯氧基)-3-(6-甲基-7-氧代-6,7-二氢- 1 H ^ -吡咯并[2,3- c ^ ]吡啶-4-基)苯基)乙磺酰胺(ABBV-075 / Mivebresib),有效且可口服的Bromodomain和Extraterminal Domain(BET)家族Bromodomain抑制剂
溴结构域和末端外结构域(BET)溴结构域抑制剂的开发及其在临床研究中,特别是在肿瘤学领域中的检查,引起了人们的广泛关注。基于简单的吡啶酮核心的制备,人们开始努力产生具有出色效能,药物代谢和药代动力学(DMPK)特性的新型BET溴结构域抑制剂。与关键的天冬酰胺残基形成双齿相互作用的努力导致掺入了吡咯并吡啶酮核心,其效价提高了9-19倍。旨在提高效力和改善药代动力学特性的额外结构-活性关系(SAR)努力导致了临床候选药物的发现63 (ABBV-075 / mivebresib),其在生化和细胞分析中显示出优异的效力,在动物模型和人类模型中均具有良好的暴露效果和半衰期,并且在癌症进展和炎症的小鼠模型中具有体内功效。






























 京公网安备 11010802027423号
京公网安备 11010802027423号