当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses.
Nature Communications ( IF 14.7 ) Pub Date : 2017-10-12 , DOI: 10.1038/s41467-017-01050-0 Guangbao Yang , Ligeng Xu , Yu Chao , Jun Xu , Xiaoqi Sun , Yifan Wu , Rui Peng , Zhuang Liu
Nature Communications ( IF 14.7 ) Pub Date : 2017-10-12 , DOI: 10.1038/s41467-017-01050-0 Guangbao Yang , Ligeng Xu , Yu Chao , Jun Xu , Xiaoqi Sun , Yifan Wu , Rui Peng , Zhuang Liu
|
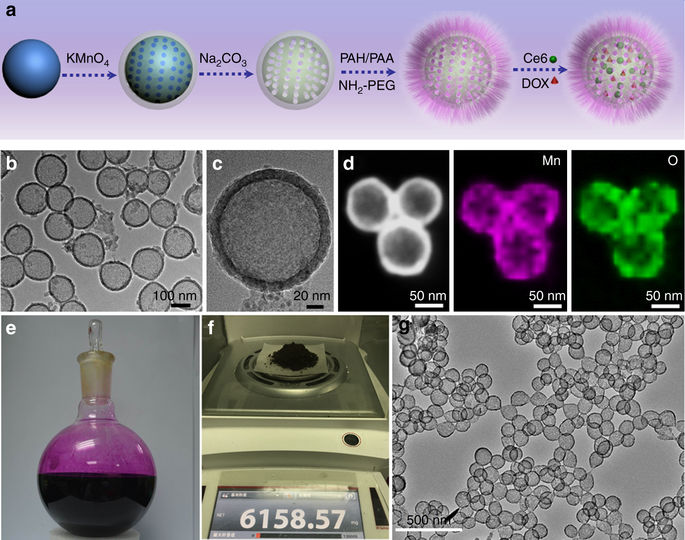
Herein, an intelligent biodegradable hollow manganese dioxide (H-MnO2) nano-platform is developed for not only tumor microenvironment (TME)-specific imaging and on-demand drug release, but also modulation of hypoxic TME to enhance cancer therapy, resulting in comprehensive effects favoring anti-tumor immune responses. With hollow structures, H-MnO2 nanoshells post modification with polyethylene glycol (PEG) could be co-loaded with a photodynamic agent chlorine e6 (Ce6), and a chemotherapy drug doxorubicin (DOX). The obtained H-MnO2-PEG/C&D would be dissociated under reduced pH within TME to release loaded therapeutic molecules, and in the meantime induce decomposition of tumor endogenous H2O2 to relieve tumor hypoxia. As a result, a remarkable in vivo synergistic therapeutic effect is achieved through the combined chemo-photodynamic therapy, which simultaneously triggers a series of anti-tumor immune responses. Its further combination with checkpoint-blockade therapy would lead to inhibition of tumors at distant sites, promising for tumor metastasis treatment.MnO2 nanostructures are promising TME-responsive theranostic agents in cancer. Here, the authors develop a nano-platform based on hollow H-MnO2 nanoshells able to modulate the tissue microenvironment, release a drug and inhibit tumor growth alone or in combination with check-point blockade therapy.
中文翻译:

中空的MnO2作为一种对肿瘤微环境有响应的可生物降解的纳米平台,用于联合治疗,有利于抗肿瘤免疫反应。
本文中,开发了一种智能的可生物降解的中空二氧化锰(H-MnO 2)纳米平台,不仅用于肿瘤微环境(TME)特定的成像和按需释放药物,还用于调节低氧TME以增强癌症治疗,从而有利于抗肿瘤免疫反应的综合作用。具有空心结构,用聚乙二醇(PEG)修饰后的H-MnO 2纳米壳可以与光动力剂氯e6(Ce6)和化疗药物阿霉素(DOX)共同装载。所获得的H-MnO 2 -PEG / C&D将在TME中在降低的pH下解离以释放负载的治疗分子,同时诱导肿瘤内源性H 2 O 2分解。缓解肿瘤缺氧。结果,通过组合的化学光动力疗法实现了显着的体内协同治疗效果,其同时触发了一系列的抗肿瘤免疫反应。它与检查点阻断疗法的进一步结合将导致远处肿瘤的抑制,有望用于肿瘤转移治疗。MnO 2纳米结构有望成为TME应答性治疗恶性肿瘤的治疗剂。在这里,作者开发了一种基于空心H-MnO 2纳米壳的纳米平台,该纳米壳能够调节组织微环境,释放药物并单独或与检查点阻断疗法结合抑制肿瘤生长。
更新日期:2017-10-12
中文翻译:

中空的MnO2作为一种对肿瘤微环境有响应的可生物降解的纳米平台,用于联合治疗,有利于抗肿瘤免疫反应。
本文中,开发了一种智能的可生物降解的中空二氧化锰(H-MnO 2)纳米平台,不仅用于肿瘤微环境(TME)特定的成像和按需释放药物,还用于调节低氧TME以增强癌症治疗,从而有利于抗肿瘤免疫反应的综合作用。具有空心结构,用聚乙二醇(PEG)修饰后的H-MnO 2纳米壳可以与光动力剂氯e6(Ce6)和化疗药物阿霉素(DOX)共同装载。所获得的H-MnO 2 -PEG / C&D将在TME中在降低的pH下解离以释放负载的治疗分子,同时诱导肿瘤内源性H 2 O 2分解。缓解肿瘤缺氧。结果,通过组合的化学光动力疗法实现了显着的体内协同治疗效果,其同时触发了一系列的抗肿瘤免疫反应。它与检查点阻断疗法的进一步结合将导致远处肿瘤的抑制,有望用于肿瘤转移治疗。MnO 2纳米结构有望成为TME应答性治疗恶性肿瘤的治疗剂。在这里,作者开发了一种基于空心H-MnO 2纳米壳的纳米平台,该纳米壳能够调节组织微环境,释放药物并单独或与检查点阻断疗法结合抑制肿瘤生长。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号