当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modulation of Amide Bond Rotamers in 5-Acyl-6,7-dihydrothieno[3,2-c]pyridines
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2015-04-09 00:00:00 , DOI: 10.1021/acs.joc.5b00205 Thomas Lanyon-Hogg 1 , Markus Ritzefeld 1 , Naoko Masumoto 1 , Anthony I Magee 2 , Henry S Rzepa 1 , Edward W Tate 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2015-04-09 00:00:00 , DOI: 10.1021/acs.joc.5b00205 Thomas Lanyon-Hogg 1 , Markus Ritzefeld 1 , Naoko Masumoto 1 , Anthony I Magee 2 , Henry S Rzepa 1 , Edward W Tate 1
Affiliation
|
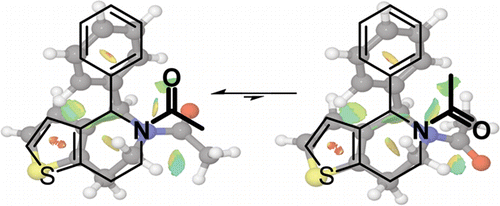
2-Substituted N-acyl-piperidine is a widespread and important structural motif, found in approximately 500 currently available structures, and present in nearly 30 pharmaceutically active compounds. Restricted rotation of the acyl substituent in such molecules can give rise to two distinct chemical environments. Here we demonstrate, using NMR studies and density functional theory modeling of the lowest energy structures of 5-acyl-6,7-dihydrothieno[3,2-c]pyridine derivatives, that the amide E:Z equilibrium is affected by non-covalent interactions between the amide oxygen and adjacent aromatic protons. Structural predictions were used to design molecules that promote either the E- or Z-amide conformation, enabling preparation of compounds with a tailored conformational ratio, as proven by NMR studies. Analysis of the available X-ray data of a variety of published N-acyl-piperidine-containing compounds further indicates that these molecules are also clustered in the two observed conformations. This finding emphasizes that directed conformational isomerism has significant implications for the design of both small molecules and larger amide-containing molecular architectures.
中文翻译:

5-酰基-6,7-二氢噻吩并[3,2-c]吡啶中酰胺键旋转异构体的调节
2-取代的N-酰基哌啶是一种广泛且重要的结构基序,在大约 500 个目前可用的结构中发现,并存在于近 30 种药物活性化合物中。此类分子中酰基取代基的受限旋转可产生两种不同的化学环境。在这里,我们使用 5-酰基-6,7-二氢噻吩并[3,2- c ]吡啶衍生物的最低能量结构的 NMR 研究和密度泛函理论模型证明,酰胺E : Z平衡受到非共价键的影响酰胺氧和相邻芳香质子之间的相互作用。结构预测用于设计促进E-或Z-酰胺构象的分子,从而能够制备具有定制构象比率的化合物,正如 NMR 研究所证明的那样。对多种已发表的含N-酰基哌啶化合物的现有 X 射线数据的分析进一步表明,这些分子也聚集在两种观察到的构象中。这一发现强调定向构象异构对于小分子和较大的含酰胺分子结构的设计具有重要意义。
更新日期:2015-04-09
中文翻译:

5-酰基-6,7-二氢噻吩并[3,2-c]吡啶中酰胺键旋转异构体的调节
2-取代的N-酰基哌啶是一种广泛且重要的结构基序,在大约 500 个目前可用的结构中发现,并存在于近 30 种药物活性化合物中。此类分子中酰基取代基的受限旋转可产生两种不同的化学环境。在这里,我们使用 5-酰基-6,7-二氢噻吩并[3,2- c ]吡啶衍生物的最低能量结构的 NMR 研究和密度泛函理论模型证明,酰胺E : Z平衡受到非共价键的影响酰胺氧和相邻芳香质子之间的相互作用。结构预测用于设计促进E-或Z-酰胺构象的分子,从而能够制备具有定制构象比率的化合物,正如 NMR 研究所证明的那样。对多种已发表的含N-酰基哌啶化合物的现有 X 射线数据的分析进一步表明,这些分子也聚集在两种观察到的构象中。这一发现强调定向构象异构对于小分子和较大的含酰胺分子结构的设计具有重要意义。































 京公网安备 11010802027423号
京公网安备 11010802027423号