当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
1(3)‐Formyl‐β‐carbolines: Potential Aldo‐X Precursors for the Synthesis of β‐Carboline‐Based Molecular Architectures
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2017-11-20 , DOI: 10.1002/ajoc.201700477 Nisha Devi 1 , Sunit Kumar 1 , Satyendra Kumar Pandey 2 , Virender Singh 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2017-11-20 , DOI: 10.1002/ajoc.201700477 Nisha Devi 1 , Sunit Kumar 1 , Satyendra Kumar Pandey 2 , Virender Singh 1
Affiliation
|
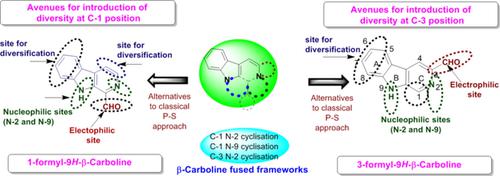
β‐Carboline, a privileged scaffold in the alkaloid family, has a broad spectrum of medicinal properties and is found in several commercial drugs, such as tadalafil, cipargamin, and abecarnil. Owing to the immense pharmacological importance of β‐carboline derivatives, their synthesis has been one of the frontier areas of research in recent years. In this context, 1(3)‐formyl‐9H‐β‐carbolines are promising “aldo‐X” bifunctional building blocks (AXB3s) that offer alternate avenues for the diversity‐oriented synthesis of β‐carboline derivatives. This Focus Review is an assimilation of recent literature (2011–2017) pertaining to the synthesis and applications of 1(3)‐formyl‐β‐carbolines for the construction of β‐carboline‐tethered and ‐fused molecular architectures. In addition, the medicinal potential of β‐carboline derivatives has been highlighted.
中文翻译:

1(3)-甲酰基-β-咔啉:合成基于β-咔啉的分子结构的潜在Aldo-X前体
β-Carboline是生物碱家族中的一种特权支架,具有广泛的医学特性,并在几种商业药物中发现,例如他达拉非,西帕加明和阿贝卡尼。由于β-咔啉衍生物的巨大药理学重要性,近年来它们的合成一直是研究的前沿领域之一。在这种情况下,1(3)-甲酰基-9 Hβ-咔啉是有前途的“ aldo-X”双功能构建基块(AXB3),可为β-咔啉衍生物的多样性导向合成提供替代途径。本《聚焦评论》是对有关1(3)-甲酰基-β-咔啉的合成和在构建β-咔啉连接和融合的分子结构中的应用的最新文献(2011-2017)的同化。此外,β-咔啉衍生物的药用潜力也得到了强调。
更新日期:2017-11-20
中文翻译:

1(3)-甲酰基-β-咔啉:合成基于β-咔啉的分子结构的潜在Aldo-X前体
β-Carboline是生物碱家族中的一种特权支架,具有广泛的医学特性,并在几种商业药物中发现,例如他达拉非,西帕加明和阿贝卡尼。由于β-咔啉衍生物的巨大药理学重要性,近年来它们的合成一直是研究的前沿领域之一。在这种情况下,1(3)-甲酰基-9 Hβ-咔啉是有前途的“ aldo-X”双功能构建基块(AXB3),可为β-咔啉衍生物的多样性导向合成提供替代途径。本《聚焦评论》是对有关1(3)-甲酰基-β-咔啉的合成和在构建β-咔啉连接和融合的分子结构中的应用的最新文献(2011-2017)的同化。此外,β-咔啉衍生物的药用潜力也得到了强调。































 京公网安备 11010802027423号
京公网安备 11010802027423号