Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2017-10-10 , DOI: 10.1016/j.cej.2017.10.020 Chaoqun Tan , Yujie Dong , Dafang Fu , Naiyun Gao , Jinxia Ma , Xiaoyu Liu
|
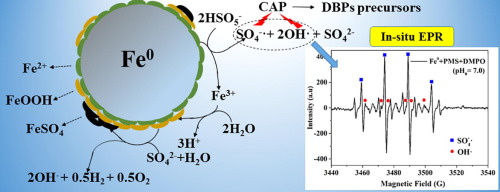
Zero valent iron (Fe0) was used as a catalyst to activate peroxymonosulfate (PMS) for removal of chloramphenicol (CAP). About 95.2% of 10 mg/L CAP was removed by 0.2 mM peroxymonosulfate and 0.5 g/L Fe0 at neutral pH, with little Fe3+ leaching. The degradation reactions well followed a pseudo-first-order kinetics pattern (R2 > 0.95). The production of hydroxyl radicals (OH) and sulfate radicals (SO4−
) was directly identified in in-situ Electron paramagnetic resonance (EPR) tests with 0.1 M 5,5-dimethyl-1-pyrrolidine N-oxide (DMPO) and indirectly identified through quenching tests. The second order rate constant between CAP and SO4−
was ascertained to be 6.18 × 1010 M−1 s−1 in competition reaction. According to SEM and XRD analysis, the average diameter of catalyst was elevated to be about 88 nm after use. Possible mechanisms on the radical generation were proposed based on the results of EPR and XPS analyses. It emerged that the cycle of Fe2+-Fe3+ on the Fe0 was answerable for the OH
and SO4−
generation, and iron sulfate layer was assembled on the Fe0 following exposure to peroxymonosulfate compared to the FeOOH layer present on the fresh Fe0. While the presence of common anions including Cl−, CO32−, Fe2+, Cu2+ and Mn2+ will inhibit the degradation of CAP in Fe0/PMS system. Besides, Fe0/PMS pre-oxidation before chlorine or chloramine engendered in a sharp increment in the concentration of disinfection by-products (DBPs) precursors. The results manifested that Fe0/PMS was effective in CAP removal at neutral pH, whereas it should be more cautiously investigated when combined with chlorine disinfection.
中文翻译:

零价铁活化过氧一硫酸盐体系脱除氯霉素的动力学和自由基生成机理
零价铁(Fe 0)用作催化剂来活化过氧单硫酸盐(PMS),以去除氯霉素(CAP)。在中性pH值下,通过0.2 mM过氧一硫酸盐和0.5 g / L Fe 0除去了约95.2%的10 mg / L CAP ,几乎没有Fe 3+的浸出。降解反应很好地遵循了拟一级反应动力学模式(R 2 > 0.95)。生产羟基自由基(OH的)和硫酸根(SO 4 -
)在原位电子顺磁共振(EPR)测试直接确定用0.1M 5,5-二甲基-1-吡咯烷-N-氧化物(DMPO)和间接通过淬火测试鉴定。CAP和SO 4之间的二阶速率常数-
在竞争反应中确定为6.18×10 10 M -1 s -1。根据SEM和XRD分析,使用后催化剂的平均直径提高到约88nm。根据EPR和XPS分析的结果,提出了可能的自由基生成机理。它涌现Fe的周期2+ -Fe 3+上的Fe 0是对此负责的OH
和SO 4 -
的生成,和硫酸铁层组装在铁0以下暴露于过氧单相比存在于所述的FeOOH层新鲜铁0。虽然常见阴离子包括氯的存在-,CO 3 2-,Fe 2 +,Cu 2+和Mn 2+会抑制Fe 0 / PMS系统中CAP的降解。此外,在消毒副产物(DBPs)前体的浓度急剧增加之前,Fe 0 / PMS在氯或氯胺之前的预氧化。结果表明,Fe 0 / PMS在中性pH下可有效去除CAP,而与氯消毒结合使用时应更加谨慎。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号