当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
What Controls Selectivity of Hydroxyl Radicals in Aqueous Solution? Indications for a Cage Effect
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-10-10 00:00:00 , DOI: 10.1021/acs.jpca.7b05782
Frank-Dieter Kopinke 1 , Anett Georgi 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-10-10 00:00:00 , DOI: 10.1021/acs.jpca.7b05782
Frank-Dieter Kopinke 1 , Anett Georgi 1
Affiliation
|
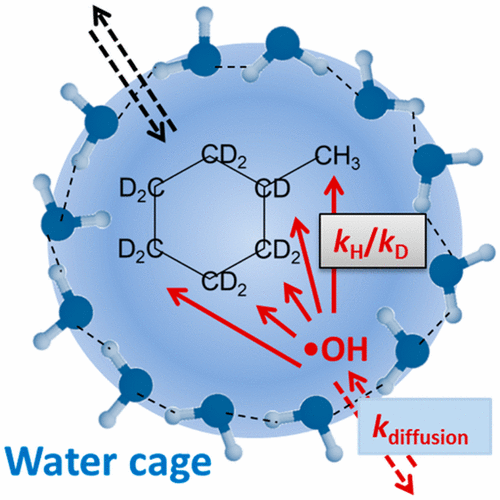
The oxidation of three isotopologues of methylcyclohexane (MCH: C7H14, C7D14, c-C6D11-CH3) by OH-radicals (•OH) in aqueous solution was investigated. Intermolecular and intramolecular H/D kinetic isotope effects (KIE = kH:kD) for the abstraction of H and D atoms by •OH were measured. These KIEs reflect inter- and intramolecular selectivities of hydrogen abstraction, i.e., the selection of •OH attack on carbon–hydrogen bonds in different molecules and in different positions of one molecule, respectively. The intermolecular selectivity of •OH attack in aqueous solution is largely discriminated against in comparison with the intramolecular selectivity. The observed extent of discrimination cannot be explained by partial diffusion control of the overall reaction rates. A cage model, where •OH and hydrocarbon molecules are entrapped in a solvent cage, is more appropriate. The much higher intramolecular KIEs compared to the intermolecular KIEs of the same chemical reaction, R–H + •OH → R• + H2O, indicate a high degree of mobility of the two reaction partners inside of the solvent cage. This mobility is sufficient to develop an intramolecular selectivity comparable to that of gas-phase reactions of •OH. Furthermore, literature data on KIEs of H-abstraction by •OH in aqueous and gas phases are discussed. There is a general tendency toward lower selectivities in the aqueous phase.
中文翻译:

什么控制着水溶液中羟基自由基的选择性?笼效应的指征
研究了水溶液中的OH自由基(• OH)对甲基环己烷(MCH:C 7 H 14,C 7 D 14,c -C 6 D 11 -CH 3)的三种同位素的氧化。测量了通过• OH提取H和D原子的分子间和分子内H / D动力学同位素效应(KIE = k H:k D)。这些KIE反映了氢提取的分子间和分子内选择性,即对氢的选择。• OH分别攻击一个分子的不同分子和不同位置的碳氢键。与分子内选择性相比,在水溶液中• OH攻击的分子间选择性大为不同。观察到的区别程度不能通过整体反应速率的部分扩散控制来解释。更适合使用笼模型,其中• OH和碳氢化合物分子被困在溶剂笼中。与相同化学反应的分子间KIE相比,分子内KIE高得多,R–H + • OH→R • + H 2O,表示溶剂笼内两个反应伙伴的高度迁移性。这种迁移率足以产生与• OH气相反应相当的分子内选择性。此外,还讨论了有关•在水相和气相中通过OH吸氢的KIE的文献资料。通常倾向于在水相中具有较低的选择性。
更新日期:2017-10-10
中文翻译:

什么控制着水溶液中羟基自由基的选择性?笼效应的指征
研究了水溶液中的OH自由基(• OH)对甲基环己烷(MCH:C 7 H 14,C 7 D 14,c -C 6 D 11 -CH 3)的三种同位素的氧化。测量了通过• OH提取H和D原子的分子间和分子内H / D动力学同位素效应(KIE = k H:k D)。这些KIE反映了氢提取的分子间和分子内选择性,即对氢的选择。• OH分别攻击一个分子的不同分子和不同位置的碳氢键。与分子内选择性相比,在水溶液中• OH攻击的分子间选择性大为不同。观察到的区别程度不能通过整体反应速率的部分扩散控制来解释。更适合使用笼模型,其中• OH和碳氢化合物分子被困在溶剂笼中。与相同化学反应的分子间KIE相比,分子内KIE高得多,R–H + • OH→R • + H 2O,表示溶剂笼内两个反应伙伴的高度迁移性。这种迁移率足以产生与• OH气相反应相当的分子内选择性。此外,还讨论了有关•在水相和气相中通过OH吸氢的KIE的文献资料。通常倾向于在水相中具有较低的选择性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号