Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthetic Nanochaperones Facilitate Refolding of Denatured Proteins
ACS Nano ( IF 15.8 ) Pub Date : 2017-10-09 00:00:00 , DOI: 10.1021/acsnano.7b05947 Fei-He Ma 1 , Yingli An 1 , Jianzu Wang 1 , Yiqing Song 1 , Yang Liu 1 , Linqi Shi 1
ACS Nano ( IF 15.8 ) Pub Date : 2017-10-09 00:00:00 , DOI: 10.1021/acsnano.7b05947 Fei-He Ma 1 , Yingli An 1 , Jianzu Wang 1 , Yiqing Song 1 , Yang Liu 1 , Linqi Shi 1
Affiliation
|
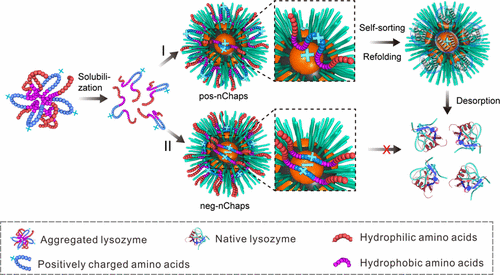
The folding process of a protein is inherently error-prone, owing to the large number of possible conformations that a protein chain can adopt. Partially folded or misfolded proteins typically expose hydrophobic surfaces and tend to form dysfunctional protein aggregates. Therefore, materials that can stabilize unfolded proteins and then efficiently assist them refolding to its bioactive form are of significant interest. Inspired by natural chaperonins, we have synthesized a series of polymeric nanochaperones that can facilitate the refolding of denatured proteins with a high recovery efficiency (up to 97%). Such nanochaperones possess phase-separated structure with hydrophobic microdomains on the surface. This structure allows nanochaperones to stabilize denatured proteins by binding them to the hydrophobic microdomains. We have also investigated the mechanism by which nanochaperones assist the protein refolding and established the design principles of nanochaperones in order to achieve effective recovery of a certain protein from their denatured forms. With a carefully designed composition of the microdomains according to the surface properties of the client proteins, the binding affinity between the hydrophobic microdomain and the denatured protein molecules can be tuned precisely, which enables the self-sorting of the polypeptides and the refolding of the proteins into their bioactive states. This work provides a feasible and effective strategy to recover inclusion bodies to their bioactive forms, which has potential to reduce the cost of the manufacture of recombinant proteins significantly.
中文翻译:

合成纳米伴侣有助于变性蛋白的折叠
由于蛋白质链可以采用大量可能的构象,因此蛋白质的折叠过程本来就容易出错。部分折叠或错误折叠的蛋白质通常会暴露疏水表面,并趋于形成功能失调的蛋白质聚集体。因此,能够稳定展开的蛋白质然后有效地帮助它们重新折叠成其生物活性形式的材料引起了人们的极大兴趣。受天然伴侣蛋白的启发,我们合成了一系列聚合纳米伴侣,可以促进变性蛋白的重折叠,回收率高(高达97%)。此类纳米分子伴侣具有相分离的结构,该结构在表面上具有疏水性微区。这种结构允许纳米分子伴侣通过将变性蛋白与疏水性微区结合来稳定变性蛋白。我们还研究了纳米分子伴侣协助蛋白质折叠的机制,并建立了纳米分子伴侣的设计原理,以便从变性形式中有效回收某些蛋白质。通过根据客户蛋白质的表面特性精心设计微区的组成,可以精确调节疏水性微区与变性蛋白质分子之间的结合亲和力,从而实现多肽的自我分选和蛋白质的重新折叠进入其生物活性状态。这项工作提供了一种可行和有效的策略,将包涵体恢复为其生物活性形式,这有可能显着降低重组蛋白的生产成本。
更新日期:2017-10-10
中文翻译:

合成纳米伴侣有助于变性蛋白的折叠
由于蛋白质链可以采用大量可能的构象,因此蛋白质的折叠过程本来就容易出错。部分折叠或错误折叠的蛋白质通常会暴露疏水表面,并趋于形成功能失调的蛋白质聚集体。因此,能够稳定展开的蛋白质然后有效地帮助它们重新折叠成其生物活性形式的材料引起了人们的极大兴趣。受天然伴侣蛋白的启发,我们合成了一系列聚合纳米伴侣,可以促进变性蛋白的重折叠,回收率高(高达97%)。此类纳米分子伴侣具有相分离的结构,该结构在表面上具有疏水性微区。这种结构允许纳米分子伴侣通过将变性蛋白与疏水性微区结合来稳定变性蛋白。我们还研究了纳米分子伴侣协助蛋白质折叠的机制,并建立了纳米分子伴侣的设计原理,以便从变性形式中有效回收某些蛋白质。通过根据客户蛋白质的表面特性精心设计微区的组成,可以精确调节疏水性微区与变性蛋白质分子之间的结合亲和力,从而实现多肽的自我分选和蛋白质的重新折叠进入其生物活性状态。这项工作提供了一种可行和有效的策略,将包涵体恢复为其生物活性形式,这有可能显着降低重组蛋白的生产成本。






























 京公网安备 11010802027423号
京公网安备 11010802027423号