当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Non-Metal-Catalyzed Heterodehydrocoupling of Phosphines and Hydrosilanes: Mechanistic Studies of B(C6F5)3-Mediated Formation of P–Si Bonds
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-11-14 , DOI: 10.1021/jacs.7b09175
Lipeng Wu 1 , Saurabh S. Chitnis 1 , Haijun Jiao 2 , Vincent T. Annibale 1 , Ian Manners 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-11-14 , DOI: 10.1021/jacs.7b09175
Lipeng Wu 1 , Saurabh S. Chitnis 1 , Haijun Jiao 2 , Vincent T. Annibale 1 , Ian Manners 1
Affiliation
|
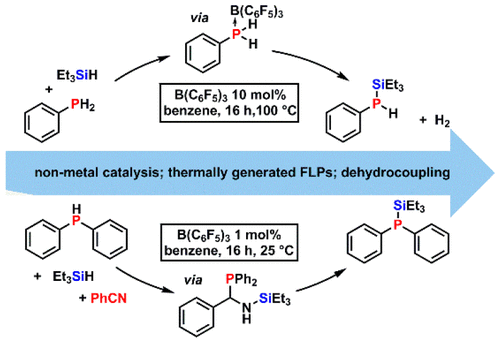
Non-metal-catalyzed heterodehydrocoupling of primary and secondary phosphines (R1R2PH, R2 = H or R1) with hydrosilanes (R3R4R5SiH, R4, R5 = H or R3) to produce synthetically useful silylphosphines (R1R2P-SiR3R4R5) has been achieved using B(C6F5)3 as the catalyst (10 mol %, 100 °C). Kinetic studies demonstrated that the reaction is first-order in hydrosilane and B(C6F5)3 but zero-order in phosphine. Control experiments, DFT calculations, and DOSY NMR studies suggest that a R1R2HP·B(C6F5)3 adduct is initially formed and undergoes partial dissociation to form an "encounter complex". The latter mediates frustrated Lewis pair type Si-H bond activation of the silane substrates. We also found that B(C6F5)3 catalyzes the homodehydrocoupling of primary phosphines to form cyclic phosphine rings and the first example of a non-metal-catalyzed hydrosilylation of P-P bonds to produce silylphosphines (R1R2P-SiR3R4R5). Moreover, the introduction of PhCN to the reactions involving secondary phosphines with hydrosilanes allowed the heterodehydrocoupling reaction to proceed efficiently under much milder conditions (1.0 mol % B(C6F5)3 at 25 °C). Mechanistic studies, as well as DFT calculations, revealed that PhCN plays a key mechanistic role in facilitating the dehydrocoupling reactions rather than simply functioning as H2-acceptor.
中文翻译:

膦和氢硅烷的非金属催化杂脱氢偶联:B(C6F5)3 介导的 P-Si 键形成的机理研究
伯膦和仲膦(R1R2PH,R2 = H 或 R1)与氢硅烷(R3R4R5SiH,R4,R5 = H 或 R3)的非金属催化杂脱氢偶联,以生产合成有用的甲硅烷基膦(R1R2P-SiR3R4R5)已使用 B(C6F5) 实现)3 作为催化剂 (10 mol%, 100 °C)。动力学研究表明该反应在氢硅烷和 B(C6F5)3 中是一级反应,而在膦中是零级反应。对照实验、DFT 计算和 DOSY NMR 研究表明,R1R2HP·B(C6F5)3 加合物最初形成并经过部分解离以形成“相遇复合物”。后者介导了硅烷基材的受挫路易斯对型 Si-H 键活化。我们还发现 B(C6F5)3 催化初级膦的同型氢偶联形成环膦环,这是非金属催化的 PP 键氢化硅烷化生成甲硅烷基膦 (R1R2P-SiR3R4R5) 的第一个例子。此外,在涉及二级膦与氢硅烷的反应中引入 PhCN 允许杂脱氢偶联反应在更温和的条件下(1.0 mol% B(C6F5)3,25°C)有效进行。机理研究以及 DFT 计算表明,PhCN 在促进脱氢偶联反应中起着关键的机械作用,而不仅仅是作为 H2 受体。将 PhCN 引入涉及二级膦与氢硅烷的反应中,允许杂脱氢偶联反应在更温和的条件下(1.0 mol% B(C6F5)3,25°C)有效进行。机理研究以及 DFT 计算表明,PhCN 在促进脱氢偶联反应中起着关键的机械作用,而不仅仅是作为 H2 受体。将 PhCN 引入涉及二级膦与氢硅烷的反应中,允许杂脱氢偶联反应在更温和的条件下(1.0 mol% B(C6F5)3,25°C)有效进行。机理研究以及 DFT 计算表明,PhCN 在促进脱氢偶联反应中起着关键的机械作用,而不仅仅是作为 H2 受体。
更新日期:2017-11-14
中文翻译:

膦和氢硅烷的非金属催化杂脱氢偶联:B(C6F5)3 介导的 P-Si 键形成的机理研究
伯膦和仲膦(R1R2PH,R2 = H 或 R1)与氢硅烷(R3R4R5SiH,R4,R5 = H 或 R3)的非金属催化杂脱氢偶联,以生产合成有用的甲硅烷基膦(R1R2P-SiR3R4R5)已使用 B(C6F5) 实现)3 作为催化剂 (10 mol%, 100 °C)。动力学研究表明该反应在氢硅烷和 B(C6F5)3 中是一级反应,而在膦中是零级反应。对照实验、DFT 计算和 DOSY NMR 研究表明,R1R2HP·B(C6F5)3 加合物最初形成并经过部分解离以形成“相遇复合物”。后者介导了硅烷基材的受挫路易斯对型 Si-H 键活化。我们还发现 B(C6F5)3 催化初级膦的同型氢偶联形成环膦环,这是非金属催化的 PP 键氢化硅烷化生成甲硅烷基膦 (R1R2P-SiR3R4R5) 的第一个例子。此外,在涉及二级膦与氢硅烷的反应中引入 PhCN 允许杂脱氢偶联反应在更温和的条件下(1.0 mol% B(C6F5)3,25°C)有效进行。机理研究以及 DFT 计算表明,PhCN 在促进脱氢偶联反应中起着关键的机械作用,而不仅仅是作为 H2 受体。将 PhCN 引入涉及二级膦与氢硅烷的反应中,允许杂脱氢偶联反应在更温和的条件下(1.0 mol% B(C6F5)3,25°C)有效进行。机理研究以及 DFT 计算表明,PhCN 在促进脱氢偶联反应中起着关键的机械作用,而不仅仅是作为 H2 受体。将 PhCN 引入涉及二级膦与氢硅烷的反应中,允许杂脱氢偶联反应在更温和的条件下(1.0 mol% B(C6F5)3,25°C)有效进行。机理研究以及 DFT 计算表明,PhCN 在促进脱氢偶联反应中起着关键的机械作用,而不仅仅是作为 H2 受体。







































 京公网安备 11010802027423号
京公网安备 11010802027423号