当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Elusive Chemistry of Hydrogen Sulfide and Mercaptans in Wine
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2017-10-09 00:00:00 , DOI: 10.1021/acs.jafc.7b02427 Vicente Ferreira 1 , Ernesto Franco-Luesma 1 , Eduardo Vela 1 , Ricardo López 1 , Purificación Hernández-Orte 1
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2017-10-09 00:00:00 , DOI: 10.1021/acs.jafc.7b02427 Vicente Ferreira 1 , Ernesto Franco-Luesma 1 , Eduardo Vela 1 , Ricardo López 1 , Purificación Hernández-Orte 1
Affiliation
|
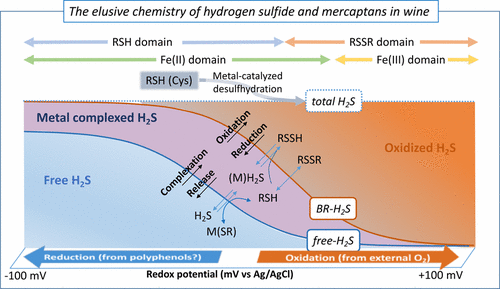
This paper summarizes, discusses, and complements recent findings about the fate of H2S and methanethiol (MeSH) during wine storage. Analytical assays to determine free volatile sulfur compounds (VSCs) and brine-releasable (BR-) VSCs in combination with accelerated reductive (AR) aging and micro-oxygenation (MOX) assays allow characterizing the different categories of species able to produce H2S and MeSH and the processes of interconversion. Each wine seems to contain a specific total amount of H2S and MeSH distributed into free, metal-complexed, and oxidized forms (di and polysulfides) interconnected through reversible redox equilibria whose external expression is wine redox potential. Oxidation transforms all mercaptans likely into nonvolatile disulfides and hydrodisulfides. In anoxia, these molecules are spontaneously and quantitatively reduced back. The concomitant accumulation of major wine thiols would provoke complex dissociation and the release of free H2S and MeSH. Additionally, total amounts can increase due to the metal-catalyzed desulfhydration of cysteine and methionine.
中文翻译:

葡萄酒中硫化氢和硫醇的难以捉摸的化学反应
本文总结,讨论并补充了有关葡萄酒储存过程中H 2 S和甲硫醇(MeSH)的命运的最新发现。结合加速还原(AR)老化和微氧化(MOX)测定的分析测定法可确定游离挥发性硫化合物(VSCs)和可释放盐水的(BR-)VSCs,可表征能够产生H 2 S的不同种类的物种和MeSH以及相互转换的过程。每种葡萄酒似乎都含有特定总量的H 2S和MeSH通过可逆的氧化还原平衡相互连接成游离的,金属络合的和氧化的形式(二硫化物和多硫化物),其外部表达是葡萄酒氧化还原电位。氧化可将所有硫醇转化为不挥发的二硫化物和氢二硫化物。在缺氧状态下,这些分子会自发并定量还原。主要葡萄酒硫醇的伴随积累会引起复杂的离解和游离H 2 S和MeSH的释放。另外,由于金属催化的半胱氨酸和蛋氨酸的脱硫,总量可能增加。
更新日期:2017-10-10
中文翻译:

葡萄酒中硫化氢和硫醇的难以捉摸的化学反应
本文总结,讨论并补充了有关葡萄酒储存过程中H 2 S和甲硫醇(MeSH)的命运的最新发现。结合加速还原(AR)老化和微氧化(MOX)测定的分析测定法可确定游离挥发性硫化合物(VSCs)和可释放盐水的(BR-)VSCs,可表征能够产生H 2 S的不同种类的物种和MeSH以及相互转换的过程。每种葡萄酒似乎都含有特定总量的H 2S和MeSH通过可逆的氧化还原平衡相互连接成游离的,金属络合的和氧化的形式(二硫化物和多硫化物),其外部表达是葡萄酒氧化还原电位。氧化可将所有硫醇转化为不挥发的二硫化物和氢二硫化物。在缺氧状态下,这些分子会自发并定量还原。主要葡萄酒硫醇的伴随积累会引起复杂的离解和游离H 2 S和MeSH的释放。另外,由于金属催化的半胱氨酸和蛋氨酸的脱硫,总量可能增加。






























 京公网安备 11010802027423号
京公网安备 11010802027423号