当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure-Based Design and Synthesis of Apramycin–Paromomycin Analogues: Importance of the Configuration at the 6′-Position and Differences between the 6′-Amino and Hydroxy Series
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-10-09 00:00:00 , DOI: 10.1021/jacs.7b07754 Appi Reddy Mandhapati 1 , Guanyu Yang 1 , Takayuki Kato 1 , Dimitri Shcherbakov 2 , Sven N. Hobbie 2 , Andrea Vasella 3 , Erik C. Böttger 2 , David Crich 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-10-09 00:00:00 , DOI: 10.1021/jacs.7b07754 Appi Reddy Mandhapati 1 , Guanyu Yang 1 , Takayuki Kato 1 , Dimitri Shcherbakov 2 , Sven N. Hobbie 2 , Andrea Vasella 3 , Erik C. Böttger 2 , David Crich 1
Affiliation
|
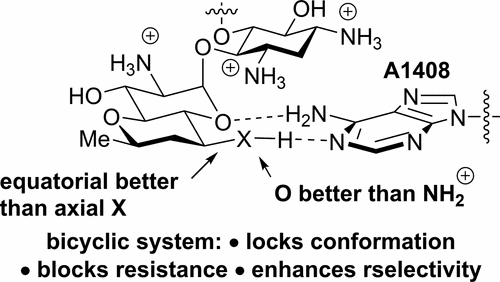
The preparation of a series of four analogues of the aminoglycoside antibiotics neomycin and paromomycin is described in which ring I, involved in critical binding interactions with the ribosomal target, is replaced by an apramycin-like dioxabicyclo[4.4.0]octane system. The effect of this modification is to lock the hydroxymethyl side chain of the neomycin or paromomycin ring I, as part of the dioxabicyclooctane ring, into either the gauche–gauche or the gauche–trans conformation (respectively, axial or equatorial to the bicyclic system). The antiribosomal activity of these compounds is investigated with cell-free translation assays using both bacterial ribosomes and recombinant hybrid ribosomes carrying eukaryotic decoding A site cassettes. Compounds substituted with an equatorial hydroxyl or amino group in the newly formed ring are considerably more active than their axial diastereomers, lending strong support to crystallographically derived models of aminoglycoside–ribosome interactions. One such bicyclic compound carrying an equatorial hydroxyl group has activity equal to that of the parent yet displays better ribosomal selectivity, predictive of an enhanced therapeutic index. A paromomycin analog lacking the hydroxymethyl ring I side chain is considerably less active than the parent. Antibacterial activity against model Gram negative and Gram positive bacteria is reported for selected compounds, as is activity against ESKAPE pathogens and recombinant bacteria carrying specific resistance determinants. Analogues with a bicyclic ring I carrying equatorial amino or hydroxyl groups mimicking the bound side chains of neomycin and paromomycin, respectively, show excellent activity and, by virtue of their novel structure, retain this activity in strains that are insensitive to the parent compounds.
中文翻译:

基于结构的设计和合成安普霉素-回蓝霉素类似物:6'-位置的构型的重要性以及6'-氨基和羟基系列之间的差异
描述了一系列氨基糖苷类抗生素新霉素和巴龙霉素的四个类似物的制备,其中参与环与核糖体靶标关键结合相互作用的环I被阿普霉素样二恶双环[4.4.0]辛烷系统取代。这种修饰的作用是将新霉素或巴龙霉素环I的羟甲基侧链(作为二氧杂双环辛烷环的一部分)锁定在gauche – gauche或gauche – trans中构象(分别为双环系统的轴向或赤道)。使用细菌核糖体和携带真核解码A位盒的重组杂种核糖体,通过无细胞翻译测定法研究了这些化合物的抗核糖体活性。在新形成的环中被赤道羟基或氨基取代的化合物比其轴向非对映异构体活性高得多,这为晶体学衍生的氨基糖苷-核糖体相互作用模型提供了有力的支持。一种这样的带有赤道羟基的双环化合物具有与母体相同的活性,但显示出更好的核糖体选择性,预示着治疗指数的提高。缺少羟甲基环I侧链的巴龙霉素类似物的活性明显低于母体。报道了针对所选化合物的针对模型革兰氏阴性和革兰氏阳性细菌的抗菌活性,以及针对ESKAPE病原体和携带特异性抗性决定簇的重组细菌的活性。具有双环I的类似物分别带有模拟赤霉素和巴龙霉素的结合侧链的赤道氨基或羟基的类似物显示出优异的活性,并且由于其新颖的结构,在对母体化合物不敏感的菌株中保留了这种活性。
更新日期:2017-10-09
中文翻译:

基于结构的设计和合成安普霉素-回蓝霉素类似物:6'-位置的构型的重要性以及6'-氨基和羟基系列之间的差异
描述了一系列氨基糖苷类抗生素新霉素和巴龙霉素的四个类似物的制备,其中参与环与核糖体靶标关键结合相互作用的环I被阿普霉素样二恶双环[4.4.0]辛烷系统取代。这种修饰的作用是将新霉素或巴龙霉素环I的羟甲基侧链(作为二氧杂双环辛烷环的一部分)锁定在gauche – gauche或gauche – trans中构象(分别为双环系统的轴向或赤道)。使用细菌核糖体和携带真核解码A位盒的重组杂种核糖体,通过无细胞翻译测定法研究了这些化合物的抗核糖体活性。在新形成的环中被赤道羟基或氨基取代的化合物比其轴向非对映异构体活性高得多,这为晶体学衍生的氨基糖苷-核糖体相互作用模型提供了有力的支持。一种这样的带有赤道羟基的双环化合物具有与母体相同的活性,但显示出更好的核糖体选择性,预示着治疗指数的提高。缺少羟甲基环I侧链的巴龙霉素类似物的活性明显低于母体。报道了针对所选化合物的针对模型革兰氏阴性和革兰氏阳性细菌的抗菌活性,以及针对ESKAPE病原体和携带特异性抗性决定簇的重组细菌的活性。具有双环I的类似物分别带有模拟赤霉素和巴龙霉素的结合侧链的赤道氨基或羟基的类似物显示出优异的活性,并且由于其新颖的结构,在对母体化合物不敏感的菌株中保留了这种活性。















































 京公网安备 11010802027423号
京公网安备 11010802027423号