Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Parkinson's disease-related DJ-1 functions in thiol quality control against aldehyde attack in vitro.
Scientific Reports ( IF 3.8 ) Pub Date : 2017-Oct-09 , DOI: 10.1038/s41598-017-13146-0
Noriyuki Matsuda , Mayumi Kimura , Bruno Barros Queliconi , Waka Kojima , Masaki Mishima , Kenji Takagi , Fumika Koyano , Koji Yamano , Tsunehiro Mizushima , Yutaka Ito , Keiji Tanaka
Scientific Reports ( IF 3.8 ) Pub Date : 2017-Oct-09 , DOI: 10.1038/s41598-017-13146-0
Noriyuki Matsuda , Mayumi Kimura , Bruno Barros Queliconi , Waka Kojima , Masaki Mishima , Kenji Takagi , Fumika Koyano , Koji Yamano , Tsunehiro Mizushima , Yutaka Ito , Keiji Tanaka
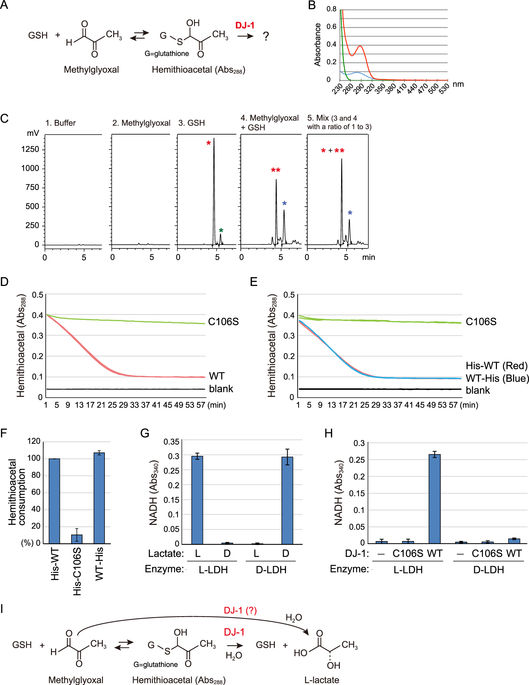
|
DJ-1 (also known as PARK7) has been identified as a causal gene for hereditary recessive Parkinson's disease (PD). Consequently, the full elucidation of DJ-1 function will help decipher the molecular mechanisms underlying PD pathogenesis. However, because various, and sometimes inconsistent, roles for DJ-1 have been reported, the molecular function of DJ-1 remains controversial. Recently, a number of papers have suggested that DJ-1 family proteins are involved in aldehyde detoxification. We found that DJ-1 indeed converts methylglyoxal (pyruvaldehyde)-adducted glutathione (GSH) to intact GSH and lactate. Based on evidence that DJ-1 functions in mitochondrial homeostasis, we focused on the possibility that DJ-1 protects co-enzyme A (CoA) and its precursor in the CoA synthetic pathway from aldehyde attack. Here, we show that intact CoA and β-alanine, an intermediate in CoA synthesis, are recovered from methylglyoxal-adducts by recombinant DJ-1 purified from E. coli. In this process, methylglyoxal is converted to L-lactate rather than the D-lactate produced by a conventional glyoxalase. PD-related pathogenic mutations of DJ-1 (L10P, M26I, A104T, D149A, and L166P) impair or abolish detoxification activity, suggesting a pathological significance. We infer that a key to understanding the biological function of DJ-1 resides in its methylglyoxal-adduct hydrolase activity, which protects low-molecular thiols, including CoA, from aldehydes.
中文翻译:

帕金森氏病相关的DJ-1在硫醇质量控制中对醛的体外攻击起作用。
DJ-1(也称为PARK7)已被确定为遗传性隐性帕金森氏病(PD)的致病基因。因此,对DJ-1功能的充分阐明将有助于破译PD发病机理的分子机制。但是,由于已经报道了DJ-1的各种作用,有时甚至是不一致的作用,因此DJ-1的分子功能仍然存在争议。近来,许多论文表明DJ-1家族蛋白与醛解毒有关。我们发现DJ-1确实将甲基乙二醛(丙酮醛)加成的谷胱甘肽(GSH)转化为完整的GSH和乳酸。基于DJ-1在线粒体体内平衡中起作用的证据,我们集中研究了DJ-1在辅酶A合成途径中保护辅酶A(CoA)及其前体免受醛攻击的可能性。这里,我们显示完整的CoA和CoA合成的中间体β-丙氨酸是通过从大肠杆菌中纯化的重组DJ-1从甲基乙二醛加合物中回收的。在该过程中,甲基乙二醛被转化为L-乳酸酯而不是由常规乙二醛酶产生的D-乳酸酯。PD相关的DJ-1致病突变(L10P,M26I,A104T,D149A和L166P)削弱或取消了排毒活性,提示其病理学意义。我们推断,了解DJ-1生物学功能的关键在于其甲基乙二醛加合物水解酶活性,该活性可保护低分子硫醇(包括CoA)免受醛的影响。甲基乙二醛被转化为L-乳酸酯,而不是由传统的乙二醛酶产生的D-乳酸酯。PD相关的DJ-1致病突变(L10P,M26I,A104T,D149A和L166P)削弱或取消了排毒活性,提示其病理学意义。我们推断,了解DJ-1生物学功能的关键在于其甲基乙二醛加合物水解酶活性,该活性可保护低分子硫醇(包括CoA)免受醛的影响。甲基乙二醛被转化为L-乳酸酯,而不是由传统的乙二醛酶产生的D-乳酸酯。PD相关的DJ-1致病突变(L10P,M26I,A104T,D149A和L166P)削弱或取消了排毒活性,提示其病理学意义。我们推断,了解DJ-1生物学功能的关键在于其甲基乙二醛加合物水解酶活性,该活性可保护低分子硫醇(包括CoA)免受醛的影响。
更新日期:2017-10-09
中文翻译:

帕金森氏病相关的DJ-1在硫醇质量控制中对醛的体外攻击起作用。
DJ-1(也称为PARK7)已被确定为遗传性隐性帕金森氏病(PD)的致病基因。因此,对DJ-1功能的充分阐明将有助于破译PD发病机理的分子机制。但是,由于已经报道了DJ-1的各种作用,有时甚至是不一致的作用,因此DJ-1的分子功能仍然存在争议。近来,许多论文表明DJ-1家族蛋白与醛解毒有关。我们发现DJ-1确实将甲基乙二醛(丙酮醛)加成的谷胱甘肽(GSH)转化为完整的GSH和乳酸。基于DJ-1在线粒体体内平衡中起作用的证据,我们集中研究了DJ-1在辅酶A合成途径中保护辅酶A(CoA)及其前体免受醛攻击的可能性。这里,我们显示完整的CoA和CoA合成的中间体β-丙氨酸是通过从大肠杆菌中纯化的重组DJ-1从甲基乙二醛加合物中回收的。在该过程中,甲基乙二醛被转化为L-乳酸酯而不是由常规乙二醛酶产生的D-乳酸酯。PD相关的DJ-1致病突变(L10P,M26I,A104T,D149A和L166P)削弱或取消了排毒活性,提示其病理学意义。我们推断,了解DJ-1生物学功能的关键在于其甲基乙二醛加合物水解酶活性,该活性可保护低分子硫醇(包括CoA)免受醛的影响。甲基乙二醛被转化为L-乳酸酯,而不是由传统的乙二醛酶产生的D-乳酸酯。PD相关的DJ-1致病突变(L10P,M26I,A104T,D149A和L166P)削弱或取消了排毒活性,提示其病理学意义。我们推断,了解DJ-1生物学功能的关键在于其甲基乙二醛加合物水解酶活性,该活性可保护低分子硫醇(包括CoA)免受醛的影响。甲基乙二醛被转化为L-乳酸酯,而不是由传统的乙二醛酶产生的D-乳酸酯。PD相关的DJ-1致病突变(L10P,M26I,A104T,D149A和L166P)削弱或取消了排毒活性,提示其病理学意义。我们推断,了解DJ-1生物学功能的关键在于其甲基乙二醛加合物水解酶活性,该活性可保护低分子硫醇(包括CoA)免受醛的影响。


































 京公网安备 11010802027423号
京公网安备 11010802027423号