当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Isothermal Vapor–Liquid Equilibria for Binary Mixtures of Methyl Nonafluorobutyl Ether + Acetone, Cyclopentyl Methyl Ether, Ethyl Acetate, n-Heptane, Methanol, and Toluene
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2017-10-09 00:00:00 , DOI: 10.1021/acs.jced.7b00599 Karel Řehák 1 , Martin Klajmon 1 , Martin Strejc 1 , Pavel Morávek 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2017-10-09 00:00:00 , DOI: 10.1021/acs.jced.7b00599 Karel Řehák 1 , Martin Klajmon 1 , Martin Strejc 1 , Pavel Morávek 1
Affiliation
|
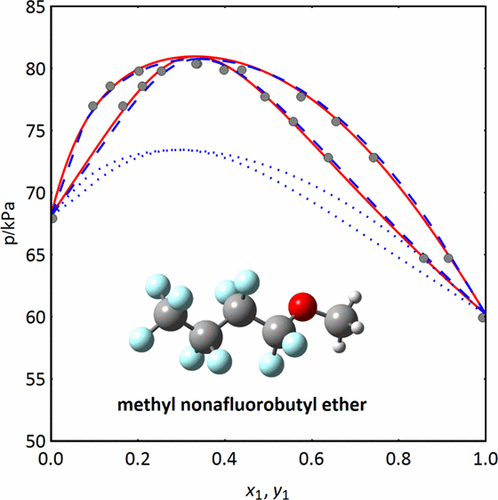
Measurements of vapor–liquid equilibrium data in binary systems methyl nonafluorobutyl ether + solvent (acetone, cyclopentyl methyl ether, ethyl acetate, n-heptane, methanol, and toluene) were carried out at constant temperature (328.15 or 318.15 K) by means of a vapor–liquid equilibrium circulation still. In the most cases, the acquired data satisfied thermodynamic consistency tests. The obtained vapor–liquid equilibrium data were (successfully) correlated by two principally different thermodynamic models: the nonrandom two-liquid equation for the excess Gibbs energy, and the perturbed-chain statistical associating fluid theory equation of state. The latter model was tested also for its predictive capabilities and was found not to be suitable for reliable predictions in the studied binary systems, although it may correctly predict the azeotropic behavior of some systems.
中文翻译:

甲基九氟丁基醚+丙酮,环戊基甲基醚,乙酸乙酯,正庚烷,甲醇和甲苯的二元混合物的等温汽液平衡
二元系统甲基九氟丁基醚+溶剂(丙酮,环戊基甲基醚,乙酸乙酯,n庚烷,甲醇和甲苯)是在恒定温度(328.15或318.15 K)下通过气-液平衡循环进行的。在大多数情况下,获得的数据满足热力学一致性测试。所获得的气液平衡数据通过两个主要不同的热力学模型(成功地)相互关联:多余吉布斯能量的非随机两液方程和状态链扰动统计关联流体理论方程。后一种模型也经过了预测能力的测试,尽管它可以正确预测某些系统的共沸行为,但不适用于所研究的二元系统中的可靠预测。
更新日期:2017-10-09
中文翻译:

甲基九氟丁基醚+丙酮,环戊基甲基醚,乙酸乙酯,正庚烷,甲醇和甲苯的二元混合物的等温汽液平衡
二元系统甲基九氟丁基醚+溶剂(丙酮,环戊基甲基醚,乙酸乙酯,n庚烷,甲醇和甲苯)是在恒定温度(328.15或318.15 K)下通过气-液平衡循环进行的。在大多数情况下,获得的数据满足热力学一致性测试。所获得的气液平衡数据通过两个主要不同的热力学模型(成功地)相互关联:多余吉布斯能量的非随机两液方程和状态链扰动统计关联流体理论方程。后一种模型也经过了预测能力的测试,尽管它可以正确预测某些系统的共沸行为,但不适用于所研究的二元系统中的可靠预测。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号