Applied Energy ( IF 10.1 ) Pub Date : 2017-10-09 , DOI: 10.1016/j.apenergy.2017.09.110
Guojie Qi , Shujuan Wang
|
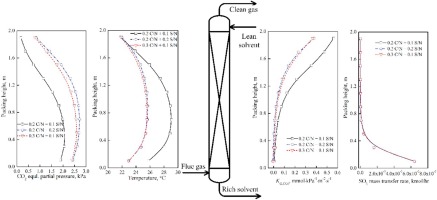
Combined CO2 and SO2 absorption performance in a new combined capture process was evaluated in this work by experimental study using a lab-scale packed column system and a developed rate-based model in Aspen Plus. The combined absorption experiments were carried out to identify the impact of several key operating parameters, including flue gas SO2 and CO2 concentrations, CO2 and SO2 loadings, the absorption temperature, and the L/G ratio, on CO2 absorption and NH3 volatilization. Experimental results indicated that ≥85% CO2 absorption efficiency was achieved in 5 wt% aqueous NH3 solvent containing lean CO2 loading at 0.2 mol CO2/mol NH3 (C/N) and SO2 loading at 0.1 mol SO2/mol NH3 (S/N). The rate-based model was developed using the electrolyte-NRTL method and the RateFrac module in Aspen Plus, and validated as compared to the present experimental results. The modeling results showed that the SO2 loading provided the similar effect on the CO2 mass transfer, the absorption efficiency and the heat of CO2 absorption as compared to the CO2 loading, however, the SO2 loading resulted in lower NH3 volatilization compared to CO2 loading. The lean CO2 loading and the solvent flow rate dynamic adjustment approaches in association with the lean NH3 solvent splitting process were studied to analyze the potential coordination of the combined absorption process and the sulfite treatment system. Maintaining 85% CO2 capture efficiency was possible when SO2 loading was increased from 0.1 to 0.2 S/N by reducing the lean CO2 loading from 0.2 to 0.088 C/N or increasing the solvent flow rate from 8 to 14.1 L/h. The time window for the SO2 treatment by the precipitation process was increased with the increasing split ratio of the lean NH3 solvent splitting process that was associated with the dynamic adjustment approaches. In the present study, the discussion of the combined absorption experiments using the CO2 and SO2 loaded NH3 solvent, the developed rate-based model, and the operation strategies provided valuable contributions to the advancement of the combined CO2 and SO2 capture process.
中文翻译:

填充塔中NH 3水溶液吸收CO 2和SO 2组合吸收的实验研究和基于速率的模型
在这项工作中,通过实验室规模的填充柱系统和Aspen Plus中基于速率的模型的实验研究,评估了在新的联合捕集过程中CO 2和SO 2的联合吸收性能。进行了联合吸收实验,以确定烟道气中的SO 2和CO 2浓度,CO 2和SO 2的负载量,吸收温度和L / G比等几个关键操作参数对CO 2吸收和吸收的影响。 NH 3挥发。实验结果表明,在5 wt%的NH 3水溶液中实现了≥85%的CO 2吸收效率包含以0.2 mol CO 2 / mol NH 3(C / N)负载的贫CO 2和以0.1 mol SO 2 / mol NH 3(S / N)负载的SO 2的稀溶剂。使用电解质-NRTL方法和Aspen Plus中的RateFrac模块开发了基于速率的模型,并与当前的实验结果进行了验证。模拟结果表明,SO 2装载设置在CO类似的效果2传质,吸收效率和CO的热2相比于CO吸收2加载,但是,SO 2加载导致较低的NH 3与CO 2负载相比挥发。研究了稀CO 2的负载和溶剂流速动态调节方法与稀NH 3溶剂裂解过程的关系,以分析组合吸收过程与亚硫酸盐处理系统之间的潜在配位关系。通过将稀薄的CO 2负载量从0.2降低到0.088 C / N或将溶剂流速从8升到14.1 L / h来将SO 2负载量从0.1增加到0.2 S / N,可以保持85%的CO 2捕集效率。随着稀NH 3分解比的增加,通过沉淀过程处理SO 2的时间窗口也增加了。与动态调整方法相关的溶剂分裂过程。在本研究中,讨论了使用负载有CO 2和SO 2的NH 3溶剂进行的联合吸收实验,已开发的基于速率的模型以及操作策略,为改进CO 2和SO 2的联合捕获提供了宝贵的贡献。过程。































 京公网安备 11010802027423号
京公网安备 11010802027423号