当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nitrosamines and Nitramines in Amine-Based Carbon Dioxide Capture Systems: Fundamentals, Engineering Implications, and Knowledge Gaps
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2017-10-06 00:00:00 , DOI: 10.1021/acs.est.7b02597 Kun Yu 1 , William A. Mitch 2 , Ning Dai 3
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2017-10-06 00:00:00 , DOI: 10.1021/acs.est.7b02597 Kun Yu 1 , William A. Mitch 2 , Ning Dai 3
Affiliation
|
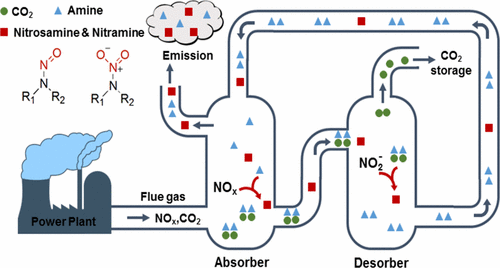
Amine-based absorption is the primary contender for postcombustion CO2 capture from fossil fuel-fired power plants. However, significant concerns have arisen regarding the formation and emission of toxic nitrosamine and nitramine byproducts from amine-based systems. This paper reviews the current knowledge regarding these byproducts in CO2 capture systems. In the absorber, flue gas NOx drives nitrosamine and nitramine formation after its dissolution into the amine solvent. The reaction mechanisms are reviewed based on CO2 capture literature as well as biological and atmospheric chemistry studies. In the desorber, nitrosamines are formed under high temperatures by amines reacting with nitrite (a hydrolysis product of NOx), but they can also thermally decompose following pseudo-first order kinetics. The effects of amine structure, primarily amine order, on nitrosamine formation and the corresponding mechanisms are discussed. Washwater units, although intended to control emissions from the absorber, can contribute to additional nitrosamine formation when accumulated amines react with residual NOx. Nitramines are much less studied than nitrosamines in CO2 capture systems. Mitigation strategies based on the reaction mechanisms in each unit of the CO2 capture systems are reviewed. Lastly, we highlight research needs in clarifying reaction mechanisms, developing analytical methods for both liquid and gas phases, and integrating different units to quantitatively predict the accumulation and emission of nitrosamines and nitramines.
中文翻译:

基于胺的二氧化碳捕集系统中的亚硝胺和硝胺:基础知识,工程意义和知识空白
胺基吸收是从化石燃料发电厂燃烧后捕获CO 2的主要竞争者。然而,对于基于胺的系统中有毒的亚硝胺和亚硝胺副产物的形成和释放已经引起了极大的关注。本文回顾了有关CO 2捕集系统中这些副产物的最新知识。在吸收器中,烟道气NO X驱动器亚硝胺和硝胺形成其溶解到胺溶剂后。基于CO 2捕获文献以及生物学和大气化学研究综述了反应机理。在解吸器中,亚硝胺是在高温下由胺与亚硝酸盐(NO x的水解产物)反应形成的。),但它们也可以按照拟一级动力学进行热分解。讨论了胺结构(主要是胺的顺序)对亚硝胺形成的影响以及相应的机理。洗涤水单元,而意在从吸收器控制的排放,可以向额外的亚硝胺的形成时积累胺与剩余的NO反应X。在CO 2捕集系统中,亚硝胺的研究远远少于亚硝胺。基于CO 2各个单元中的反应机理的缓解策略捕获系统进行了审查。最后,我们着重指出了在澄清反应机理,开发液相和气相分析方法以及整合不同单元以定量预测亚硝胺和亚硝胺的积累和排放方面的研究需求。
更新日期:2017-10-07
中文翻译:

基于胺的二氧化碳捕集系统中的亚硝胺和硝胺:基础知识,工程意义和知识空白
胺基吸收是从化石燃料发电厂燃烧后捕获CO 2的主要竞争者。然而,对于基于胺的系统中有毒的亚硝胺和亚硝胺副产物的形成和释放已经引起了极大的关注。本文回顾了有关CO 2捕集系统中这些副产物的最新知识。在吸收器中,烟道气NO X驱动器亚硝胺和硝胺形成其溶解到胺溶剂后。基于CO 2捕获文献以及生物学和大气化学研究综述了反应机理。在解吸器中,亚硝胺是在高温下由胺与亚硝酸盐(NO x的水解产物)反应形成的。),但它们也可以按照拟一级动力学进行热分解。讨论了胺结构(主要是胺的顺序)对亚硝胺形成的影响以及相应的机理。洗涤水单元,而意在从吸收器控制的排放,可以向额外的亚硝胺的形成时积累胺与剩余的NO反应X。在CO 2捕集系统中,亚硝胺的研究远远少于亚硝胺。基于CO 2各个单元中的反应机理的缓解策略捕获系统进行了审查。最后,我们着重指出了在澄清反应机理,开发液相和气相分析方法以及整合不同单元以定量预测亚硝胺和亚硝胺的积累和排放方面的研究需求。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号